Polyamine: Difference between revisions
m →Biological function: Task 17: replace to-be-deprecated: |name-list-format= (1× replaced; usage: 1 of 8); |
rm "interesting" x2 |
||
| (34 intermediate revisions by 23 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Type of organic compound}} |
|||
{{distinguish|diamine}} |
{{distinguish|diamine}} |
||
A '''polyamine''' is an [[organic compound]] having more than two [[amino group]]s. Alkyl polyamines occur naturally but |
A '''polyamine''' is an [[organic compound]] having more than two [[amino group]]s. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, [[Hygroscopy|hygroscopic]], and water soluble. Near neutral pH, they exist as the ammonium derivatives.<ref name=Ullmann>{{Ullmann|first1=Karsten|last1=Eller|first2=Erhard|last2=Henkes|first3=Roland|last3=Rossbacher|first4=Hartmut|last4=Höke|title=Amines, Aliphatic|year=2005|doi=10.1002/14356007.a02_001}}</ref> Most [[aromatic compound|aromatic]] polyamines are crystalline solids at room temperature. |
||
==Natural polyamines== |
==Natural polyamines== |
||
Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine |
Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine [[spermidine]] and the tetraamine [[spermine]]. They are structurally and biosynthetically related to the diamines [[putrescine]] and [[cadaverine]]. Polyamine metabolism is regulated by the activity of the enzyme [[ornithine decarboxylase]] (ODC).<ref name=Pegg>{{cite journal| title=Polyamine metabolism and function| first1=AE| last1=Pegg| first2=PP| last2=McCann| journal=[[American Journal of Physiology]]| year=1982| volume=243| issue=5| pages=212–221| pmid=6814260| doi=10.1152/ajpcell.1982.243.5.C212| s2cid=21063248}}</ref> Polyamines are found in high concentrations in the [[mammal]]ian [[brain]].<ref name=Seiler>{{cite book |first=N. |last=Seiler |chapter=Polyamines |chapter-url=https://link.springer.com/chapter/10.1007/978-1-4757-0614-7_9 |editor-last=Lajtha |editor-first=A. |title=Chemical and Cellular Architecture |series=Handbook of Neurochemistry |volume=1 |publisher=Springer |edition=2nd |date=1982 |isbn=978-1-4757-0614-7 |pages=223–255 |doi=10.1007/978-1-4757-0614-7_9}}</ref> |
||
<gallery caption="Natural polyamines" widths="180px" heights="120px" |
<gallery caption="Natural polyamines" widths="180px" heights="120px"> |
||
File:Spermidine-2D-skeletal. |
File:Spermidine-2D-skeletal.svg|[[spermidine]] |
||
File:Spermine.svg|[[spermine]] |
File:Spermine.svg|[[spermine]] |
||
</gallery> |
</gallery> |
||
==Synthetic polyamines== |
==Synthetic polyamines== |
||
{{see also|Ethyleneamines}} |
|||
Ethyleneamines are a commercially-important class of synthetic polyamines with ethylene ({{chem2|\sCH2CH2\s}} linkages); global production capacity was estimated at 385,000 tonnes in 2001.<ref name = kirk>{{Kirk-Othmer | title = Diamines and Higher Amines, Aliphatic | author1 = Srivasan Sridhar | author2 = Richard G. Carter | doi = 10.1002/0471238961.0409011303011820.a01.pub2 | year = 2001 | mode = cs1}}</ref> They are chemical intermediates often used to make surfactants and as crosslinkers for [[epoxy]] resins.<ref name=lawr>{{cite book |first=Stephen A. |last=Lawrence |title=Amines: synthesis, properties and applications |url=https://books.google.com/books?id=35xwBwjhe2MC&pg=PA64 |year=2004 |publisher=Cambridge University Press |isbn=978-0-521-78284-5 |page=64}} |
|||
</ref> Many synthetic polyamines feature NCH<sub>2</sub>CH<sub>2</sub>N linkages: |
|||
</ref> Some members of this class include: |
|||
*[[Diethylenetriamine]], abbreviated dien or DETA, (H<sub>2</sub>N-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH<sub>2</sub>. The related permethylated derivative [[pentamethyldiethylenetriamine]] is used as a chelating agent in [[organolithium chemistry]]. |
|||
* [[Ethylenediamine]], first member of this series. It is a chelating ligand by itself, and it is a precursor to the popular metal sequestrant, [[EDTA]] (ethylenediaminetetraacetic acid). Permethylated, ethylenediamine yields [[tetramethylethylenediamine]] (TMEDA) that has a very high affinity for lithium ions.<ref name=EROS>{{cite encyclopedia |last1=Haynes |first1=R. K. |last2=Vonwiller |first2=S. C. |last3=Luderer |first3=M. R. | title = ''N'',''N'',''N''′,''N''′-Tetramethylethylenediamine | encyclopedia = Encyclopedia of Reagents for Organic Synthesis | editor-last = Paquette |editor-first=L. | year = 2006 | publisher = Wiley | doi = 10.1002/047084289X.rt064.pub2 |chapter=N,N,N′,N′-Tetramethylethylenediamine |isbn=0-471-93623-5}}</ref> |
|||
*[[Triethylenetetramine]] (trien or TETA, H<sub>2</sub>N-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH<sub>2</sub>), tetraethylenepentamine (TEPA, H<sub>2</sub>N-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH<sub>2</sub>), pentaethylenehexamine (PEHA, H<sub>2</sub>N-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH-CH<sub>2</sub>CH<sub>2</sub>-NH<sub>2</sub>). |
|||
*[[Macrocycle|Macrocyclic]] polyamines: [[1,4,7-Triazacyclononane|1,4,7-triazacyclononane]] (( |
*[[Macrocycle|Macrocyclic]] polyamines analogous to [[crown ethers]]: [[1,4,7-Triazacyclononane|1,4,7-triazacyclononane]] ({{chem2|(NHCH2CH2)3}}) and [[cyclen]] ({{chem2|(NHCH2CH2)4}}). A related tetraaza macrocycle is [[cyclam]]. |
||
*[[Tris(2-aminoethyl)amine]] (N( |
*[[Tris(2-aminoethyl)amine]] ({{chem2|N(CH2CH2NH2)3}}) is a branched polyamine that is a minor side product of the [[polyethyleneamine]] process. A related tripodal polyamine is [[1,1,1-Tris(aminomethyl)ethane|1,1,1-tris(aminomethyl)ethane]]. These are chelating ligands. |
||
| ⚫ | |||
| ⚫ | |||
Other synthetic polyamines include [[1,3,5-triazinane]] (not to be confused with [[1,3,5-triazine]]) and N-substituted analogs. The methylene ({{chem2|\sCH2}}) linkages are derived from [[formaldehyde]]. The reaction product of monoethanolamine and formaldehyde is known industrially as "MEA triazine" (it is actually a triazinane), and it serves as a water-soluble [[hydrogen sulfide]] scavenger.<ref>{{cite conference | title = Fresh Insight into the H2S Scavenging Mechanism of MEA-Triazine vs. MMA-Triazine | author1 = G. N. Taylor | author2 = J. J. Wylde | author3 = T. Müller | author4 = J Murison | author5 = F. Schneider | conference = SPE International Conference on Oilfield Chemistry | location = Montgomery, Texas | year = 2017 | number = SPE-184529-MS | doi = 10.2118/184529-MS}}</ref> [[Hexamethylenetetramine]] (hexamine) is another product of formaldehyde and ammonia that has various uses in industry. Domestically, it is used as a solid camping fuel. In the laboratory, it reacts with [[alkyl halides]] to selectively prepare primary amines in the [[Delépine reaction]]. |
|||
| ⚫ | |||
File:Tris(2-aminoethyl)amine.svg|[[Tris(2-aminoethyl)amine]] |
File:Tris(2-aminoethyl)amine.svg|[[Tris(2-aminoethyl)amine]] |
||
File:Cyclen.svg|[[Cyclen]] |
File:Cyclen.svg|[[Cyclen]] |
||
File:1,4,7-triazacyclononane. |
File:1,4,7-triazacyclononane.svg|[[1,4,7-Triazacyclononane]] |
||
File:1,1,1-Tris(aminomethyl)ethane.svg|[[1,1,1-Tris(aminomethyl)ethane]] |
File:1,1,1-Tris(aminomethyl)ethane.svg|[[1,1,1-Tris(aminomethyl)ethane]] |
||
File:Branched PEI.png|Subunit of [[polyethylenimine]] |
File:Branched PEI.png|Subunit of [[polyethylenimine]] |
||
File:Hexamine.svg|[[Hexamethylenetetramine]] with its [[adamantane]]-type structure |
|||
</gallery> |
</gallery> |
||
| ⚫ | |||
==Biological function== |
==Biological function== |
||
Although it is known that the biosynthesis of polyamines is highly regulated, the biological function of polyamines is only partly |
Although it is known that the biosynthesis of polyamines is highly regulated, the biological function of polyamines is only partly understood. In their cationic ammonium form, they bind to [[DNA]], and, in structure, they represent compounds with cations that are found at ''regularly spaced intervals'' (in contrast to {{chem|Mg|2+|link=Mg ion (physiology)}} or {{chem|Ca|2+|link=Calcium in biology}}, which are point charges). They have also been found to act as promoters of programmed ribosomal frameshifting during translation.<ref>{{cite journal|author1=Rato C |author2=Amirova S.R |author3=Bates D.G |author4=Stansfield I |author5=Wallace H.M |title=Translational recoding as a feedback controller: systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift|journal=Nucleic Acids Res.|volume=39|issue=11|pages=4587–97|date=June 2011|doi=10.1093/nar/gkq1349|pmid=21303766|pmc=3113565}}</ref> |
||
Inhibition of polyamine biosynthesis |
Inhibition of polyamine biosynthesis retards or stops [[cell growth]]. The provision of exogenous polyamines restores the growth of these cells. Most eukaryotic cells express a [[polyamine-transporting ATPase]] on their [[cell membrane]] that facilitates the internalization of exogenous polyamines. This system is highly active in rapidly proliferating cells and is the target of some chemotherapeutics currently under development.<ref>{{cite journal |vauthors=Wang C, Delcros JG, Cannon L, Konate F, Carias H, Biggerstaff J, Gardner RA, Phanstiel IV O |title=Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters |journal=J Med Chem |volume=46 |issue=24 |pages=5129–38 |date=November 2003 |pmid=14613316 |doi=10.1021/jm030223a }}</ref> |
||
Polyamines are also modulators of a variety of [[ion channel]]s, including [[NMDA receptor]]s and [[AMPA receptor]]s. They block [[inward-rectifier potassium channel]]s so that the currents of the channels are inwardly rectified, thereby the cellular energy, i.e. {{chem|K|+}} ion gradient across the cell membrane, is conserved. In addition, polyamine participate in initiating the expression of SOS response of Colicin E7 operon and down-regulate proteins that are essential for colicin E7 uptake, thus conferring a survival advantage on colicin-producing ''E. coli'' under stress conditions.<ref>{{cite journal |author1=Yi-Hsuan Pan |author2=Chen-Chung Liao |title=The critical roles of polyamines regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli|journal=J. Biol. Chem. |volume=281|pages= |
Polyamines are also modulators of a variety of [[ion channel]]s, including [[NMDA receptor]]s and [[AMPA receptor]]s. They block [[inward-rectifier potassium channel]]s so that the currents of the channels are inwardly rectified, thereby the cellular energy, i.e. {{chem|K|+}} ion gradient across the cell membrane, is conserved. In addition, polyamine participate in initiating the expression of SOS response of Colicin E7 operon and down-regulate proteins that are essential for colicin E7 uptake, thus conferring a survival advantage on colicin-producing ''E. coli'' under stress conditions.<ref>{{cite journal |author1=Yi-Hsuan Pan |author2=Chen-Chung Liao |title=The critical roles of polyamines regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli|journal=J. Biol. Chem. |volume=281|pages=13083–91 |date=May 2006 |pmid=16549429 |issue=19 |doi=10.1074/jbc.M511365200| doi-access=free }}</ref> |
||
Polyamines can enhance the permeability of the [[blood–brain barrier]].<ref>{{cite journal |vauthors=Zhang L, Lee HK, Pruess TH, White HS, Bulaj G |title=Synthesis and applications of polyamine amino acid residues: improving the bioactivity of an analgesic neuropeptide, neurotensin |journal=J. Med. Chem. |volume=52 |issue=6 |pages=1514–7 |date=March 2009 |pmid=19236044 |pmc=2694617 |doi=10.1021/jm801481y }}</ref> |
Polyamines can enhance the permeability of the [[blood–brain barrier]].<ref>{{cite journal |vauthors=Zhang L, Lee HK, Pruess TH, White HS, Bulaj G |title=Synthesis and applications of polyamine amino acid residues: improving the bioactivity of an analgesic neuropeptide, neurotensin |journal=J. Med. Chem. |volume=52 |issue=6 |pages=1514–7 |date=March 2009 |pmid=19236044 |pmc=2694617 |doi=10.1021/jm801481y }}</ref> |
||
They are involved in modulating [[senescence]] of organs in plants and are therefore considered as a [[plant hormone]].<ref>{{cite journal |vauthors=Pandey S, Ranade SA, Nagar PK, Kumar N |title=Role of polyamines and ethylene as modulators of plant senescence |journal=J. Biosci. |volume=25 |issue=3 |pages=291–9 |date=September 2000 |pmid=11022232 |doi=10.1007/BF02703938}}</ref> In addition, they are directly involved in regulation of programmed cell death.<ref>{{cite journal|last=Moschou|first=PN|author2=Roubelakis-Angelakis, KA|title=Polyamines and programmed cell death.|journal=Journal of Experimental Botany|date=Nov 11, 2013|pmid=24218329|doi=10.1093/jxb/ert373|volume=65| issue=5 |pages= |
They are involved in modulating [[senescence]] of organs in plants and are therefore considered as a [[plant hormone]].<ref>{{cite journal |vauthors=Pandey S, Ranade SA, Nagar PK, Kumar N |title=Role of polyamines and ethylene as modulators of plant senescence |journal=J. Biosci. |volume=25 |issue=3 |pages=291–9 |date=September 2000 |pmid=11022232 |doi=10.1007/BF02703938|s2cid=21925829 }}</ref> In addition, they are directly involved in regulation of programmed cell death.<ref>{{cite journal|last=Moschou|first=PN|author2=Roubelakis-Angelakis, KA|title=Polyamines and programmed cell death.|journal=Journal of Experimental Botany|date=Nov 11, 2013|pmid=24218329|doi=10.1093/jxb/ert373|volume=65| issue=5 |pages=1285–96|doi-access=free}}</ref> |
||
===Homology-directed DNA repair=== |
|||
Polyamines promote [[homologous recombination]] (HR)-mediated [[DNA repair|double-strand break (DSB) repair]].<ref name = Lee2019>{{cite journal |vauthors=Lee CY, Su GC, Huang WY, Ko MY, Yeh HY, Chang GD, Lin SJ, Chi P |title=Promotion of homology-directed DNA repair by polyamines |journal=Nat Commun |volume=10 |issue=1 |pages=65 |date=January 2019 |pmid=30622262 |pmc=6325121 |doi=10.1038/s41467-018-08011-1 |bibcode=2019NatCo..10...65L }}</ref> Polyamines enhance the DNA strand exchange activity of [[RAD51]] [[recombinase]]. Depletion of polyamines sensitizes cells to [[genotoxicity|genotoxic substances]] such as [[ionizing radiation]] and [[ultraviolet]] radiation. The effect of polyamines on RAD51 arises from their ability to enhance the capture of homologous duplex DNA and promote RAD-51-mediated homologous DNA pairing and exchange activity.<ref name = Lee2019/> Polyamines appear to have an evolutionarily conserved role in regulating recombinase activity. |
|||
==Biosynthesis of spermidine, spermine, thermospermine== |
==Biosynthesis of spermidine, spermine, thermospermine== |
||
[[File:Polyamine synthesis. |
[[File:Polyamine synthesis.svg|thumb|400x400px|Biosynthesis of spermidine and spermine from putrescine. Ado = 5'-adenosyl]] |
||
[[Spermidine]] is synthesized from putrescine, using an aminopropyl group from decarboxylated [[S-adenosyl-L-methionine|''S''-adenosyl-L-methionine]] (SAM). The reaction is catalyzed by [[spermidine synthase]]. |
[[Spermidine]] is synthesized from putrescine, using an aminopropyl group from decarboxylated [[S-adenosyl-L-methionine|''S''-adenosyl-L-methionine]] (SAM), [[S-Adenosylmethioninamine|''S''-Adenosylmethioninamine]]. The reaction is catalyzed by [[spermidine synthase]].<ref name="pmid26089148">{{cite journal | vauthors = Pál M, Szalai G, Janda T | title = Speculation: Polyamines are important in abiotic stress signaling | journal = [[Plant Science (journal)|Plant Science]] | volume = 237 | pages = 16–23 |date=2015 | doi = 10.1016/j.plantsci.2015.05.003 | pmid = 26089148| bibcode = 2015PlnSc.237...16P | url = http://real.mtak.hu/24396/1/Uneditted.pdf }}</ref> |
||
[[Spermine]] is synthesized from the reaction of spermidine with SAM in the presence of the enzyme [[spermine synthase]]. |
[[Spermine]] is synthesized from the reaction of spermidine with SAM in the presence of the enzyme [[spermine synthase]]. |
||
The polyamines undergo rapid interconversion in the polyamine cycle, in which putrescine leads to synthesis of spermidine and spermine, with degradation of these polyamines to form putrescine, which can begin the cycle again.<ref name="pmid26089148" /> |
|||
{{Clear}} |
{{Clear}} |
||
Thermospermine ( |
{{anchor|Thermospermine}}Thermospermine ({{chem2|NH2\s(CH2)3\sNH\s(CH2)3\sNH\s(CH2)4\sNH2}}) is a structural [[isomer]] of spermine and a plant growth regulator. It is produced from spermidine by the action of [[thermospermine synthase]], which is encoded by a gene named ACAULIS5 (ACL5).<ref name=Takeno>{{cite journal| title=Thermospermine is not a minor polyamine in the plant kingdom | journal= Plant Cell Physiol| date=April 2012| volume=53| issue=4| pages=606–16| doi=10.1093/pcp/pcs019| pmid=22366038| last1 = Takano | first1 = A | last2 = Kakehi | first2 = J | last3 = Takahashi | first3 = T| url= https://zenodo.org/record/851716| doi-access=free}}</ref> |
||
==Polyamine analogues== |
==Polyamine analogues== |
||
The critical role of polyamines in cell growth has led to the development of a number of agents that interfere with polyamine metabolism. These agents are used in cancer therapy. Polyamine analogues upregulate [[p53]] in a cell leading to restriction of proliferation and apoptosis.<ref>{{cite journal|title=Role of p53/p21(Waf1/Cip1) in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells|journal=Cancer Biology & Therapy|volume=4|issue=9|pages= |
The critical role of polyamines in cell growth has led to the development of a number of agents that interfere with polyamine metabolism. These agents are used in [[cancer therapy]]. Polyamine analogues upregulate [[p53]] in a cell leading to restriction of proliferation and [[apoptosis]].<ref>{{cite journal|title=Role of p53/p21(Waf1/Cip1) in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells|journal=Cancer Biology & Therapy|volume=4|issue=9|pages=1006–13|date=September 2005|last1=Huang|first1=Yi|last2=Pledgie|first2=Allison|last3=Rubin|first3=Ethel| last4=Marton|first4=Laurence J.|last5=Woster|first5=Patrick M.|last6=Sukumar|first6=Saraswati|last7=Casero|first7=Robert A.|last8=Davidson|first8=Nancy E.|pmc=3639297|doi=10.4161/cbt.4.9.1970|pmid=16131835}}</ref> It also decreases the expression of estrogen receptor alpha in ER-positive breast cancer.<ref>{{cite journal|title=Polyamine analogues down-regulate estrogen receptor alpha expression in human breast cancer cells| pmid=16679312 | doi= 10.1074/jbc.M600910200 | volume=281| issue=28 | pmc=3623667| journal=J Biol Chem| pages=19055–63 | last1 = Huang | first1 = Y | last2 = Keen | first2 = JC | last3 = Pledgie | first3 = A | last4 = Marton | first4 = LJ | last5 = Zhu | first5 = T | last6 = Sukumar | first6 = S | last7 = Park | first7 = BH | last8 = Blair | first8 = B | last9 = Brenner | first9 = K | last10 = Casero | first10 = RA Jr | last11 = Davidson | first11 = NE| year=2006| doi-access=free }}</ref> |
||
==See also== |
|||
*[[Polyamine oxidase]] |
|||
*[[Polyamines in plant stress]] |
|||
*[[Polyamine-modulated factor 1]] |
|||
==References== |
==References== |
||
| Line 55: | Line 69: | ||
==External links== |
==External links== |
||
*[https://web.archive.org/web/20130420091712/http://www4.lu.se/cell-proliferation-group/research/the-role-of-the-polyamines-in-cell-cycle-control-and-program Polyamines in cell cycle proliferation and cell death] |
*[https://web.archive.org/web/20130420091712/http://www4.lu.se/cell-proliferation-group/research/the-role-of-the-polyamines-in-cell-cycle-control-and-program Polyamines in cell cycle proliferation and cell death] |
||
* |
*{{cite book |first=Pekka |last=Kilpeläinen |title=Expression and regulation in rat brain and in transgenic mice |publisher=Department of Biochemistry, University of Oulu |date=2002 |isbn=951-42-6631-5 |hdl=10024/37387 |url=https://oulurepo.oulu.fi/handle/10024/37387}} Extensive review of literature through 2001 on polyamine structure, properties, metabolism in mammals, and physiological and pathophysiological roles (See article Table of Contents) |
||
{{Plant_hormones}} |
{{Plant_hormones}} |
||
{{Authority control}} |
|||
[[Category:Polyamines| ]] |
[[Category:Polyamines| ]] |
||
Latest revision as of 03:38, 18 November 2024
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives.[1] Most aromatic polyamines are crystalline solids at room temperature.
Natural polyamines
[edit]Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC).[2] Polyamines are found in high concentrations in the mammalian brain.[3]
- Natural polyamines
Synthetic polyamines
[edit]Ethyleneamines are a commercially-important class of synthetic polyamines with ethylene (−CH2CH2− linkages); global production capacity was estimated at 385,000 tonnes in 2001.[4] They are chemical intermediates often used to make surfactants and as crosslinkers for epoxy resins.[5] Some members of this class include:
- Ethylenediamine, first member of this series. It is a chelating ligand by itself, and it is a precursor to the popular metal sequestrant, EDTA (ethylenediaminetetraacetic acid). Permethylated, ethylenediamine yields tetramethylethylenediamine (TMEDA) that has a very high affinity for lithium ions.[6]
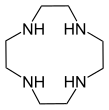
- Macrocyclic polyamines analogous to crown ethers: 1,4,7-triazacyclononane ((NHCH2CH2)3) and cyclen ((NHCH2CH2)4). A related tetraaza macrocycle is cyclam.
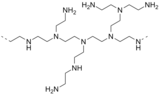
- Tris(2-aminoethyl)amine (N(CH2CH2NH2)3) is a branched polyamine that is a minor side product of the polyethyleneamine process. A related tripodal polyamine is 1,1,1-tris(aminomethyl)ethane. These are chelating ligands.
- Polyethylenimine is a polymer derived from aziridine.
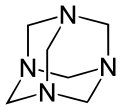
Other synthetic polyamines include 1,3,5-triazinane (not to be confused with 1,3,5-triazine) and N-substituted analogs. The methylene (−CH2) linkages are derived from formaldehyde. The reaction product of monoethanolamine and formaldehyde is known industrially as "MEA triazine" (it is actually a triazinane), and it serves as a water-soluble hydrogen sulfide scavenger.[7] Hexamethylenetetramine (hexamine) is another product of formaldehyde and ammonia that has various uses in industry. Domestically, it is used as a solid camping fuel. In the laboratory, it reacts with alkyl halides to selectively prepare primary amines in the Delépine reaction.
- Synthetic polyamines
-
Subunit of polyethylenimine
-
Hexamethylenetetramine with its adamantane-type structure
Biological function
[edit]Although it is known that the biosynthesis of polyamines is highly regulated, the biological function of polyamines is only partly understood. In their cationic ammonium form, they bind to DNA, and, in structure, they represent compounds with cations that are found at regularly spaced intervals (in contrast to Mg2+
or Ca2+
, which are point charges). They have also been found to act as promoters of programmed ribosomal frameshifting during translation.[8]
Inhibition of polyamine biosynthesis retards or stops cell growth. The provision of exogenous polyamines restores the growth of these cells. Most eukaryotic cells express a polyamine-transporting ATPase on their cell membrane that facilitates the internalization of exogenous polyamines. This system is highly active in rapidly proliferating cells and is the target of some chemotherapeutics currently under development.[9]
Polyamines are also modulators of a variety of ion channels, including NMDA receptors and AMPA receptors. They block inward-rectifier potassium channels so that the currents of the channels are inwardly rectified, thereby the cellular energy, i.e. K+
ion gradient across the cell membrane, is conserved. In addition, polyamine participate in initiating the expression of SOS response of Colicin E7 operon and down-regulate proteins that are essential for colicin E7 uptake, thus conferring a survival advantage on colicin-producing E. coli under stress conditions.[10]
Polyamines can enhance the permeability of the blood–brain barrier.[11]
They are involved in modulating senescence of organs in plants and are therefore considered as a plant hormone.[12] In addition, they are directly involved in regulation of programmed cell death.[13]
Homology-directed DNA repair
[edit]Polyamines promote homologous recombination (HR)-mediated double-strand break (DSB) repair.[14] Polyamines enhance the DNA strand exchange activity of RAD51 recombinase. Depletion of polyamines sensitizes cells to genotoxic substances such as ionizing radiation and ultraviolet radiation. The effect of polyamines on RAD51 arises from their ability to enhance the capture of homologous duplex DNA and promote RAD-51-mediated homologous DNA pairing and exchange activity.[14] Polyamines appear to have an evolutionarily conserved role in regulating recombinase activity.
Biosynthesis of spermidine, spermine, thermospermine
[edit]
Spermidine is synthesized from putrescine, using an aminopropyl group from decarboxylated S-adenosyl-L-methionine (SAM), S-Adenosylmethioninamine. The reaction is catalyzed by spermidine synthase.[15]
Spermine is synthesized from the reaction of spermidine with SAM in the presence of the enzyme spermine synthase.
The polyamines undergo rapid interconversion in the polyamine cycle, in which putrescine leads to synthesis of spermidine and spermine, with degradation of these polyamines to form putrescine, which can begin the cycle again.[15]
Thermospermine (NH2−(CH2)3−NH−(CH2)3−NH−(CH2)4−NH2) is a structural isomer of spermine and a plant growth regulator. It is produced from spermidine by the action of thermospermine synthase, which is encoded by a gene named ACAULIS5 (ACL5).[16]
Polyamine analogues
[edit]The critical role of polyamines in cell growth has led to the development of a number of agents that interfere with polyamine metabolism. These agents are used in cancer therapy. Polyamine analogues upregulate p53 in a cell leading to restriction of proliferation and apoptosis.[17] It also decreases the expression of estrogen receptor alpha in ER-positive breast cancer.[18]
See also
[edit]References
[edit]- ^ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 978-3527306732.
- ^ Pegg, AE; McCann, PP (1982). "Polyamine metabolism and function". American Journal of Physiology. 243 (5): 212–221. doi:10.1152/ajpcell.1982.243.5.C212. PMID 6814260. S2CID 21063248.
- ^ Seiler, N. (1982). "Polyamines". In Lajtha, A. (ed.). Chemical and Cellular Architecture. Handbook of Neurochemistry. Vol. 1 (2nd ed.). Springer. pp. 223–255. doi:10.1007/978-1-4757-0614-7_9. ISBN 978-1-4757-0614-7.
- ^ Srivasan Sridhar; Richard G. Carter (2001). "Diamines and Higher Amines, Aliphatic". Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley. doi:10.1002/0471238961.0409011303011820.a01.pub2. ISBN 9780471238966.
- ^ Lawrence, Stephen A. (2004). Amines: synthesis, properties and applications. Cambridge University Press. p. 64. ISBN 978-0-521-78284-5.
- ^ Haynes, R. K.; Vonwiller, S. C.; Luderer, M. R. (2006). "N,N,N′,N′-Tetramethylethylenediamine". In Paquette, L. (ed.). N,N,N′,N′-Tetramethylethylenediamine. Encyclopedia of Reagents for Organic Synthesis. Wiley. doi:10.1002/047084289X.rt064.pub2. ISBN 0-471-93623-5.
- ^ G. N. Taylor; J. J. Wylde; T. Müller; J Murison; F. Schneider (2017). Fresh Insight into the H2S Scavenging Mechanism of MEA-Triazine vs. MMA-Triazine. SPE International Conference on Oilfield Chemistry. Montgomery, Texas. doi:10.2118/184529-MS.
- ^ Rato C; Amirova S.R; Bates D.G; Stansfield I; Wallace H.M (June 2011). "Translational recoding as a feedback controller: systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift". Nucleic Acids Res. 39 (11): 4587–97. doi:10.1093/nar/gkq1349. PMC 3113565. PMID 21303766.
- ^ Wang C, Delcros JG, Cannon L, Konate F, Carias H, Biggerstaff J, Gardner RA, Phanstiel IV O (November 2003). "Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters". J Med Chem. 46 (24): 5129–38. doi:10.1021/jm030223a. PMID 14613316.
- ^ Yi-Hsuan Pan; Chen-Chung Liao (May 2006). "The critical roles of polyamines regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli". J. Biol. Chem. 281 (19): 13083–91. doi:10.1074/jbc.M511365200. PMID 16549429.
- ^ Zhang L, Lee HK, Pruess TH, White HS, Bulaj G (March 2009). "Synthesis and applications of polyamine amino acid residues: improving the bioactivity of an analgesic neuropeptide, neurotensin". J. Med. Chem. 52 (6): 1514–7. doi:10.1021/jm801481y. PMC 2694617. PMID 19236044.
- ^ Pandey S, Ranade SA, Nagar PK, Kumar N (September 2000). "Role of polyamines and ethylene as modulators of plant senescence". J. Biosci. 25 (3): 291–9. doi:10.1007/BF02703938. PMID 11022232. S2CID 21925829.
- ^ Moschou, PN; Roubelakis-Angelakis, KA (Nov 11, 2013). "Polyamines and programmed cell death". Journal of Experimental Botany. 65 (5): 1285–96. doi:10.1093/jxb/ert373. PMID 24218329.
- ^ a b Lee CY, Su GC, Huang WY, Ko MY, Yeh HY, Chang GD, Lin SJ, Chi P (January 2019). "Promotion of homology-directed DNA repair by polyamines". Nat Commun. 10 (1): 65. Bibcode:2019NatCo..10...65L. doi:10.1038/s41467-018-08011-1. PMC 6325121. PMID 30622262.
- ^ a b Pál M, Szalai G, Janda T (2015). "Speculation: Polyamines are important in abiotic stress signaling" (PDF). Plant Science. 237: 16–23. Bibcode:2015PlnSc.237...16P. doi:10.1016/j.plantsci.2015.05.003. PMID 26089148.
- ^ Takano, A; Kakehi, J; Takahashi, T (April 2012). "Thermospermine is not a minor polyamine in the plant kingdom". Plant Cell Physiol. 53 (4): 606–16. doi:10.1093/pcp/pcs019. PMID 22366038.
- ^ Huang, Yi; Pledgie, Allison; Rubin, Ethel; Marton, Laurence J.; Woster, Patrick M.; Sukumar, Saraswati; Casero, Robert A.; Davidson, Nancy E. (September 2005). "Role of p53/p21(Waf1/Cip1) in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells". Cancer Biology & Therapy. 4 (9): 1006–13. doi:10.4161/cbt.4.9.1970. PMC 3639297. PMID 16131835.
- ^ Huang, Y; Keen, JC; Pledgie, A; Marton, LJ; Zhu, T; Sukumar, S; Park, BH; Blair, B; Brenner, K; Casero, RA Jr; Davidson, NE (2006). "Polyamine analogues down-regulate estrogen receptor alpha expression in human breast cancer cells". J Biol Chem. 281 (28): 19055–63. doi:10.1074/jbc.M600910200. PMC 3623667. PMID 16679312.
External links
[edit]- Polyamines in cell cycle proliferation and cell death
- Kilpeläinen, Pekka (2002). Expression and regulation in rat brain and in transgenic mice. Department of Biochemistry, University of Oulu. hdl:10024/37387. ISBN 951-42-6631-5. Extensive review of literature through 2001 on polyamine structure, properties, metabolism in mammals, and physiological and pathophysiological roles (See article Table of Contents)