Balz–Schiemann reaction
Appearance
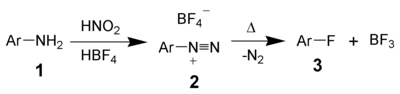
The Schiemann reaction (also called the Balz–Schiemann reaction) is a chemical reaction in which anilines (1) are transformed to aryl fluorides (3) via diazonium fluoroborates (2).[1][2] Named after the German chemists Günther Schiemann and Günther Balz, this reaction is the preferred route to fluorobenzene and some related derivatives,[3] including 4-fluorobenzoic acid.[4]
The reaction is similar to the Sandmeyer reaction, which converts diazonium salts to other aryl halides.[5]
References
- ^ Günther Balz, Günther Schiemann (1927). "Über aromatische Fluorverbindungen, I.: Ein neues Verfahren zu ihrer Darstellung". Ber. 5 (60): 1186–1190. doi:10.1002/cber.19270600539.
- ^ Roe, A. Org. React. 1949, 5, 193. (Review)
- ^ Flood, D. T. (1943). "Fluorobenzene". Organic Syntheses; Collected Volumes, vol. 2, p. 295.
- ^ G. Schiemann; W. Winkelmüller (1943). "p-Fluorobenzoic Acid". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 2, p. 299. - ^ Swain, C. G.; Rogers, R. J. (1975). "Mechanism of formation of aryl fluorides from arenediazonium fluoborates". J. Am. Chem. Soc. 97: 799–800. doi:10.1021/ja00837a019.
{{cite journal}}: CS1 maint: multiple names: authors list (link)