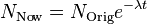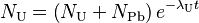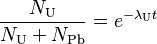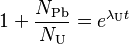Uranium–lead dating
Uranium–lead (U–Pb) dating is one of the oldest[1] and most refined of the radiometric dating schemes. It can be used to date rocks that formed from about 1 million years to over 4.5 billion years ago with routine precisions in the 0.1–1 percent range.[2]
The uranium–lead dating method relies on two separate decay chains, the uranium series from 238U to 206Pb, with a half-life of 4.47 billion years and the actinium series from 235U to 207Pb, with a half-life of 710 million years.
These uranium to lead decay routes occur via a series of alpha (and beta) decays, in which 238U with daughter nuclides undergo eight total alpha and six beta decays whereas 235U with daughters only experience seven alpha and four beta decays.[3]
The existence of two 'parallel' uranium-lead decay routes (238U to 206Pb and 235U to 207Pb) leads to multiple dating techniques within the overall U–Pb system. The term U–Pb dating normally implies the coupled use of both decay schemes in the 'concordia diagram' (see below).
However, use of a single decay scheme (usually 238U to 206Pb) leads to the U–Pb isochron dating method, analogous to the rubidium-strontium dating method.
Finally, ages can also be determined from the U–Pb system by analysis of Pb isotope ratios alone. This is termed the lead-lead dating method. Clair Cameron Patterson, an American geochemist who pioneered studies of uranium–lead radiometric dating methods, is famous for having used it to obtain one of the earliest accurate estimates of the age of the Earth.
Contents
Mineralogy
Uranium-lead dating is usually performed on the mineral zircon (ZrSiO4), though it can be used on other minerals such as monazite, titanite, and baddeleyite.
The zircon mineral incorporates uranium and thorium atoms into its crystalline structure, but strongly rejects lead. Therefore, we can assume that the entire lead content of the zircon is radiogenic.
Where crystals such as zircon with uranium and thorium inclusions do not occur, a better, more inclusive, model of the data must be applied. Uranium-lead dating techniques have also been applied to other minerals such as calcite/aragonite and other carbonate minerals. These types of minerals often produce lower precision ages than igneous and metamorphic minerals traditionally used for age dating, but are more common in the geologic record.
Interaction between mineralogy and radioactive breakdown
During the alpha decay steps, the zircon crystal experiences radiation damage, associated with each alpha decay. This damage is most concentrated around the parent isotope (U and Th), expelling the daughter isotope (Pb) from its original position in the zircon lattice.
In areas with a high concentration of the parent isotope, damage to the crystal lattice is quite extensive, and will often interconnect to form a network of radiation damaged areas.[3] Fission tracks and micro-cracks within the crystal will further extend this radiation damage network.
These fission tracks inevitably act as conduits deep within the crystal, thereby providing a method of transport to facilitate the leaching of lead isotopes from the zircon crystal.[4]
Chemical details
Under conditions where no lead loss or gain from the outside environment has occurred, the age of the zircon rock can be calculated by the equations for exponential decay like so:
where in this situation:
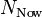 is the number of Uranium atoms measured now.
is the number of Uranium atoms measured now.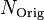 is the number of Uranium atoms originally - equal to the sum of Lead and Uranium atoms measured now.
is the number of Uranium atoms originally - equal to the sum of Lead and Uranium atoms measured now.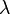 is the decay rate of Uranium.
is the decay rate of Uranium.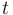 is the age of the zircon rock, which one wants to determine.
is the age of the zircon rock, which one wants to determine.
The above equation is thus equal to:
Which can be rearranged:
and simplified:
The more common form of the equations finally are:
-
-
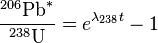
(1)
-
and
-
-
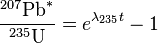
(2)
-
These are said to yield concordant ages. It is these concordant ages, plotted over a series of time intervals, that result in the concordant line.[5]
Loss (leakage) of lead from the sample will result in a discrepancy in the ages determined by each decay scheme. This effect is referred to as discordance and is demonstrated in Figure 1. If a series of zircon samples has lost different amounts of lead, the samples generate a discordant line. The upper intercept of the concordia and the discordia line will reflect the original age of formation, while the lower intercept will reflect the age of the event that led to open system behavior and therefore the lead loss; although there has been some disagreement regarding the meaning of the lower intercept ages.[5]
Undamaged zircon retains the lead generated by radioactive decay of uranium and thorium until very high temperatures (about 900 °C), though accumulated radiation damage within zones of very high uranium can lower this temperature substantially. Zircon is very chemically inert and resistant to mechanical weathering—a mixed blessing for geochronologists, as zones or even whole crystals can survive melting of their parent rock with their original uranium-lead age intact. Zircon crystals with prolonged and complex histories can thus contain zones of dramatically different ages (usually, with the oldest and youngest zones forming the core and rim, respectively, of the crystal), and thus are said to demonstrate inherited characteristics. Unraveling such complications (which, depending on their maximum lead-retention temperature, can also exist within other minerals) generally requires in situ micro-beam analysis via, say, ion microprobe (SIMS) or laser ICP-MS.
See also
- Lead-lead dating (Pb-Pb dating)
References
| The Wikibook Historical Geology has a page on the topic of: Other isochron methods |
| The Wikibook Historical Geology has a page on the topic of: U-Pb, Pb-Pb, and fission track dating |
- ↑ Boltwood, B.B., 1907, On the ultimate disintegration products of the radio-active elements. Part II. The disintegration products of uranium: American Journal of Science 23: 77-88.
- ↑ Parrish, Randall R.; Noble, Stephen R., 2003. Zircon U-Th-Pb Geochronology by Isotope Dilution – Thermal Ionization Mass Spectrometry (ID-TIMS). In Zircon (eds. J. Hanchar and P. Hoskin). Reviews in Mineralogy and Geochemistry, Mineralogical Society of America. 183-213.
- ↑ 3.0 3.1 Romer, R.L. 2003. Alpha-recoil in U-Pb geochronology: Effective sample size matters. Contributions to Mineralogy and Petrology 145, (4): 481-491.
- ↑ 4.0 4.1 Mattinson, J.M., 2005. Zircon U-Pb Chemical abrasion (“CA-TIMS”) method: Combined annealing and multi-step dissolution analysis for Improved precision and accuracy of zircon ages. Chemical Geology. 220, 47-66.
- ↑ 5.0 5.1 Dickin, A.P., 2005. Radiogenic Isotope Geology 2nd ed. Cambridge: Cambridge University Press. pp. 101.