Abstract
Type 2 diabetes, obesity, and sex difference affect myocardial glucose uptake and utilization. However, their effect on the intramyocellular fate of glucose in humans has been unknown. How the heart uses glucose is important, because it affects energy production and oxygen efficiency, which in turn affect heart function and adaptability. We hypothesized that type 2 diabetes, sex difference, and obesity affect myocardial glucose oxidation, glycolysis, and glycogen production. In a first-in-human study, we measured intramyocardiocellular glucose metabolism from time-activity curves generated from previously obtained positron emission tomography scans of 110 subjects in 3 groups: nonobese, obese, and diabetes. Group and sex difference interacted in the prediction of all glucose uptake, utilization, and metabolism rates. Group independently predicted fractional glucose uptake and its components: glycolysis, glycogen deposition, and glucose oxidation rates. Sex difference predicted glycolysis rates. However, there were fewer differences in glucose metabolism between diabetic patients and others when plasma glucose levels were included in the modeling. The potentially detrimental effects of obesity and diabetes on myocardial glucose metabolism are more pronounced in men than women. This sex difference dimorphism needs to be taken into account in the design, trials, and application of metabolic modulator therapy. Slightly higher plasma glucose levels improve depressed glucose oxidation and glycogen deposition rates in diabetic patients.
Keywords: type 2 diabetes, obesity, sex difference, glucose metabolism, myocardium, glucose oxidation
type 2 diabetes [type 2 diabetes mellitus (T2DM)] and obesity are major risk factors for cardiac dysfunction and heart failure (12, 14). Myocardial function is inextricably linked to substrate metabolism. Under normal aerobic conditions, 50–70% of total energy is obtained from oxidation of fatty acids; the rest is primarily derived from carbohydrates (glucose and lactate). The proportional contribution of these various substrates to myocardial energy metabolism is exquisitely sensitive to the substrate environment, hormonal milieu, level of myocardial work, and level of myocardial blood flow (MBF). For example, under fasting conditions, myocardial fatty acid metabolism is the predominant energy source. However, glucose is a more oxygen-efficient fuel per carbon than fatty acids, but only if fully oxidized.
In the diabetic human heart, glucose uptake is decreased (18) and glucose utilization/plasma insulin is lower than in nondiabetic controls (19). Animal models of diabetes and obesity demonstrate impaired glucose uptake from reduced insulin-mediated translocation of the GLUT4 transporter (3). In murine models of diabetes and obesity, glycolysis and glucose oxidation rates are also decreased (3). However, the effects of diabetes and obesity on glycolysis, glucose oxidation, and glycogen deposition are unknown in humans.
Sex difference also plays a major role in determination of glucose uptake and utilization by the heart in nonobese (21), obese (20), and diabetic subjects (17). The effect of sex difference on intramyocardial glucose metabolism in the human heart is not known. Thus we hypothesized that group (diabetes, obese, and nonobese) and sex difference would affect intracellular glucose metabolism. To test our hypothesis, we quantified glycolysis, glucose oxidation, and glycogen deposition rates in the human heart. We utilized time-activity curves generated from previously performed positron emission tomography (PET) scans in nonobese, obese, and diabetic subjects, but we applied new, well-validated, compartmental modeling for quantification of intramyocardiocellular glucose metabolism (9).
MATERIALS AND METHODS
Subjects
Patients (n = 108) were divided into three groups: nonobese [n = 10, body mass index (BMI) <30 kg/m2], obese (n = 26, BMI >30.0 kg/m2), and T2DM (n = 72). A medical history was obtained from each patient, and clinical assessment, physical examination, and routine blood chemistries were performed. Excluded from the study were women with a lack of adequate birth control and patients with Hb A1c >7.0, serum triglycerides >400 mg/dl, hypertension beyond stage 1, or a history of coronary artery disease or any other cardiac disease (except mild valvular disease), including left ventricular (LV) wall motion abnormalities. All patients were nonsmokers and had no other systemic illnesses.
The T2DM group also underwent rest/stress echocardiography to rule out coronary artery disease. No patient was taking niacin, fibrate, thiazolidinedione, or insulin; 40% were taking statins, and 43% were taking antihypertensive medication. The Human Studies Committee and the Radioactive Drug Research Committee at the Washington University School of Medicine approved the study. All patients signed informed consent prior to enrollment in the study.
Positron Emission Tomography
All PET studies were performed using a Siemens tomograph (ECAT 962 HR+, Siemens Medical Systems, Iselin, NJ). Patients were fasted for 12 h prior to the study. Two intravenous catheters, one for infusion and one for blood sampling, were placed. All studies were carried out at 0800 to avoid circadian variations in metabolism. Blood pressure and heart rate were monitored throughout the study.
PET Image Analysis
Myocardial H215O and [1-11C]glucose images were obtained for measurements to determine MBF and glucose metabolism, respectively. Well-established kinetic models were used in conjunction with PET-generated time-activity curves to quantify MBF (10) and glucose metabolism (9). Calculations were based on models described previously using rate constants to determine glucose uptake by the cells and, ultimately, the mitochondria. The following equations are used to describe calculation of the glucose metabolism measurements: fractional myocardial glucose uptake per gram of heart muscle (ml·g−1·min−1) = glucose extraction fraction × MBF (ml·g−1·min−1); fractional myocardial glucose uptake = glucose for glycolysis + glucose for glycogen storage; portion of fractional glucose uptake undergoing glycolysis = portion undergoing full glucose oxidation + lactate production; myocardial glucose utilization (nmol·g−1·min−1) = fractional glucose uptake × plasma glucose concentration at the time of the PET scan. Glucose utilization was also subdivided on the basis of the kinetic modeling into glycolysis + glycogen storage rates. The portion of glycolysis that was attributable to full glucose oxidation was also determined using the modeling referenced above.
Echocardiography
All patients underwent a complete resting echocardiogram (Sequoia-C256, Acuson-Siemens, Mountain View, CA) during their PET study, following their scan for MBF, and before their scan for myocardial glucose metabolism. Two-dimensional echocardiograms were performed and analyzed according to American Society of Echocardiography guidelines (15). The area-length method was used for measurement of LV mass. LV end-diastolic and end-systolic volumes were measured in the four-chamber view, and the modified Simpson's equation was used to calculate ejection fraction.
Measurement of Plasma Insulin and Substrates
Plasma insulin was measured by radioimmunoassay (Linco Research, St. Charles, MO). Plasma glucose and lactate levels were measured using a glucose-lactate analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma free fatty acid (FFA) levels were measured using an enzymatic kit (NEFA C kit, WAKO Chemicals USA, Richmond, VA).
Statistical Methods
GraphPad Prism 6.05 (GraphPad Software, San Diego, CA) was used to perform all statistical analyses. Data are presented as means ± SD. Analysis of variance was used to determine differences in continuous variables across the three groups: nonobese, obese, and T2DM. χ2 analysis was used for categorical variables. Two-way analysis of variance was used to determine the independent effects of group and sex difference and their interaction in determination of our predetermined end points, myocardial glucose fractional uptake, utilization, and their component parts, i.e., glycogen deposition and glycolysis, as well as the portion of glycolysis accounted for by glucose oxidation. Post hoc testing to analyze paired comparisons was done using Fisher's least significant difference test.
RESULTS
Table 1 shows the baseline characteristics of the patients, their plasma lipid and insulin levels, and MBF rates at the time of the PET study. There was no difference in the percentage of women in the groups. The T2DM patients were well controlled on the basis of average Hb A1c. Age and BMI differed across the three groups: patients in the T2DM group were the oldest, and those in the obese group had the highest BMI. Plasma insulin levels were highest in the obese and T2DM groups (Table 1). MBF was also different among the groups: MBF was highest in the obese group (Table 1). Plasma glucose levels were different among the groups: glucose levels were highest in the T2DM group (Fig. 1). There was also no significant interaction between sex difference and group in the determination of plasma FFA concentration, which was higher (P < 0.05) in the obese and T2DM groups than in the nonobese group and higher (P < 0.001) in women than in men (Fig. 1).
Table 1.
Baseline characteristics, plasma levels, and blood flow
| Group |
|||
|---|---|---|---|
| Nonobese (n = 10) | Obese (n = 26) | T2DM (n = 72) | |
| Age, yr | 31 ± 9 | 35 ± 6 | 56 ± 9†††,‡‡‡ |
| Sex, %women | 60 | 65 | 55 |
| BMI, kg/m2 | 23 ± 1 | 38 ± 5*** | 34 ± 7†††,‡‡‡ |
| Plasma insulin, μU/ml | 4.9 ± 3.0 | 13.3 ± 6.1* | 14.5 ± 10.4†† |
| Hb A1c | 6.6 ± 0.7 | ||
| Total cholesterol, mg/dl | 155 ± 16 | 169 ± 29 | 149 ± 30‡‡ |
| Low-density lipoprotein, mg/dl | 82 ± 20 | 95 ± 26 | 75 ± 23‡‡‡ |
| High-density lipoprotein, mg/dl | 57 ± 13 | 46 ± 11** | 45 ± 11†† |
| Triglycerides, mg/dl | 78 ± 26 | 141 ± 76** | 140 ± 63†† |
| MBF, ml·g−1·min−1 | 0.97 ± 0.12 | 1.18 ± 0.38* | 1.04 ± 0.24‡ |
Values are means ± SD.
BMI, body mass index; MBF, myocardial blood flow.
Post hoc comparisons of groups:
P < 0.05, obese vs. nonobese;
P < 0.01, obese vs. nonobese;
P < 0.001, obese vs. nonobese; †P < 0.05, T2DM vs. nonobese;
P < 0.01, T2DM vs. nonobese;
P < 0.001, T2DM vs. nonobese;
P < 0.05, T2DM vs. obese;
P < 0.01, T2DM vs. obese;
P < 0.001, T2DM vs. obese.
Fig. 1.
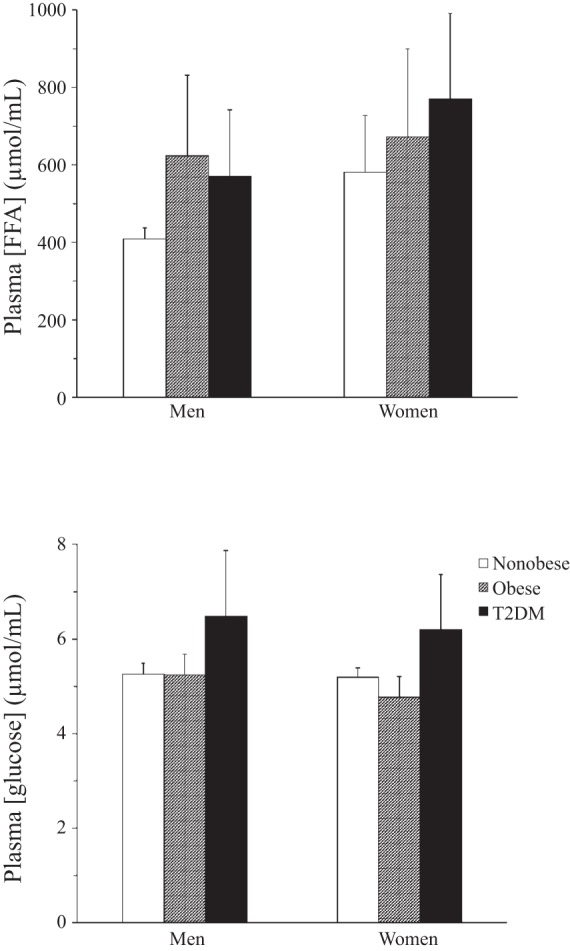
Plasma free fatty acid (FFA) and glucose concentrations in men and women. T2DM, type 2 diabetes mellitus. Plasma glucose concentration was significantly higher (P < 0.001) in diabetic than nondiabetic subjects, but there was no difference between men and women and there were no significant interaction effects. Plasma FFA concentration was significantly higher (P < 0.05) in obese and T2DM groups than nonobese group and also significantly higher (P < 0.001) in women than men, but there was no significant interaction effect.
Hemodynamics and Cardiac Structure and Function
Hemodynamic and cardiac structure and function data are shown in Table 2. Resting heart rate differed among the three groups: rates were higher in the obese and T2DM groups than in the nonobese group. Consistent with this heart rate difference, ejection fraction was highest in the T2DM group. Systolic blood pressure and ejection fraction were higher in the T2DM than nonobese group. Diastolic blood pressure and LV mass were not different among the groups. Rate-pressure product, a surrogate of cardiac work, was highest in the T2DM group. However, despite this evidence of higher work (and higher ejection fraction), fractional glucose uptake was not higher in the T2DM group (see below and Fig. 2).
Table 2.
Hemodynamics and cardiac structure and function
| Group |
|||
|---|---|---|---|
| Nonobese | Obese | T2DM | |
| Heart rate, beats/min | 56 ± 8 | 68 ± 11** | 67 ± 10†† |
| SBP, mmHg | 116 ± 16 | 126 ± 12 | 131 ± 17†† |
| DBP, mmHg | 65 ± 8 | 69 ± 8 | 68 ± 7 |
| Rate-pressure product, (beats·min−1)·mmHg | 6,581 ± 1,526 | 8,591 ± 1,724** | 8,698 ± 1,863††† |
| LV mass, g | 150 ± 50 | 181 ± 35 | 173 ± 45 |
| Ejection fraction, % | 58 ± 5 | 61 ± 5 | 64 ± 6† |
Values are means ± SD.
SBP and DBP, systolic and diastolic blood pressure; LV, left ventricular.
Post hoc comparisons of groups:
P < 0.01, obese vs. nonobese;
P < 0.05, T2DM vs. nonobese;
P < 0.01, T2DM vs. nonobese;
P < 0.001, T2DM vs. nonobese.
Fig. 2.
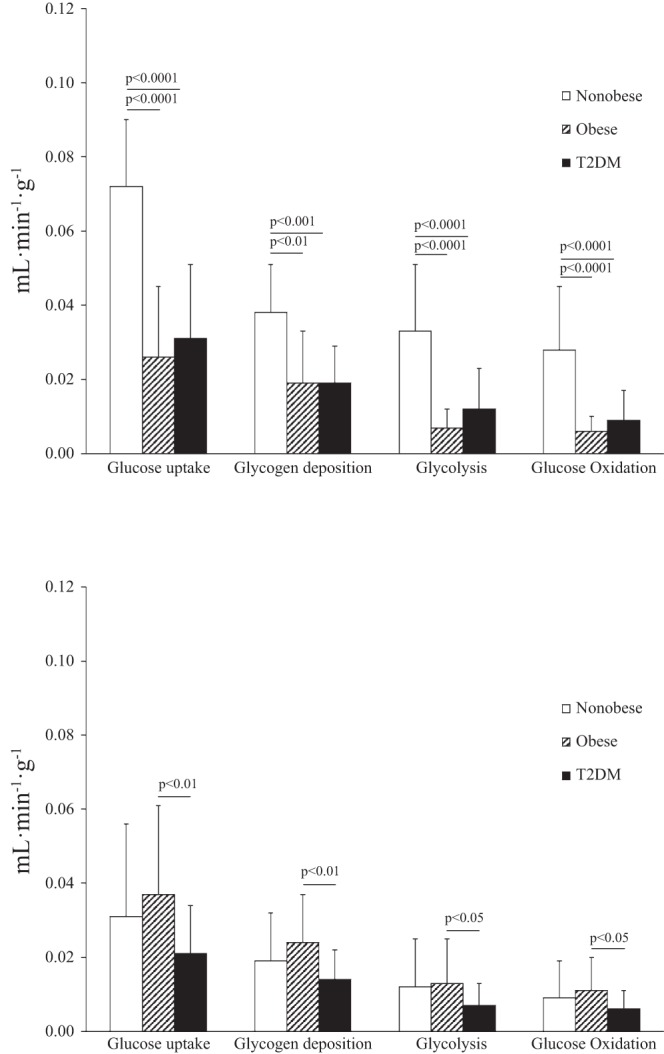
Fractional myocardial glucose uptake and its components in men (top) and women (bottom).
Fractional Glucose Uptake and Intramyocardial Glucose Kinetics
Men.
Fractional glucose uptake was markedly higher in nonobese men than obese or T2DM men (Fig. 2, top). Similar patterns were observed for the portion of fractional glucose uptake that underwent glycolysis, glycogen deposition, and glucose oxidation.
Women.
The pattern of fractional glucose uptake and metabolism among the groups differed between women and men. The difference in fractional glucose uptake among the groups was due to higher glucose uptake in obese than T2DM women. Similarly, glycogen deposition, glycolysis, and glucose oxidation rates were higher in obese than T2DM women.
Glucose Utilization and Intramyocardial Glucose Kinetics
Men.
Among the three groups of men, glucose utilization patterns were similar to glucose uptake patterns (Fig. 3, top). Glucose utilization was higher in nonobese than obese or T2DM men. The portion of glucose utilization accounted for by glycogen deposition, glycolysis, and glucose oxidation was also higher in nonobese than obese or T2DM men. The significance of the difference (P value) in glucose utilization between nonobese and T2DM men is less than the difference in fractional glucose uptake between these same two groups (Figs. 2 and 3). This is likely due to the higher plasma glucose levels in T2DM men (Fig. 1), because glucose utilization is the product of fractional glucose uptake and plasma glucose concentration.
Fig. 3.
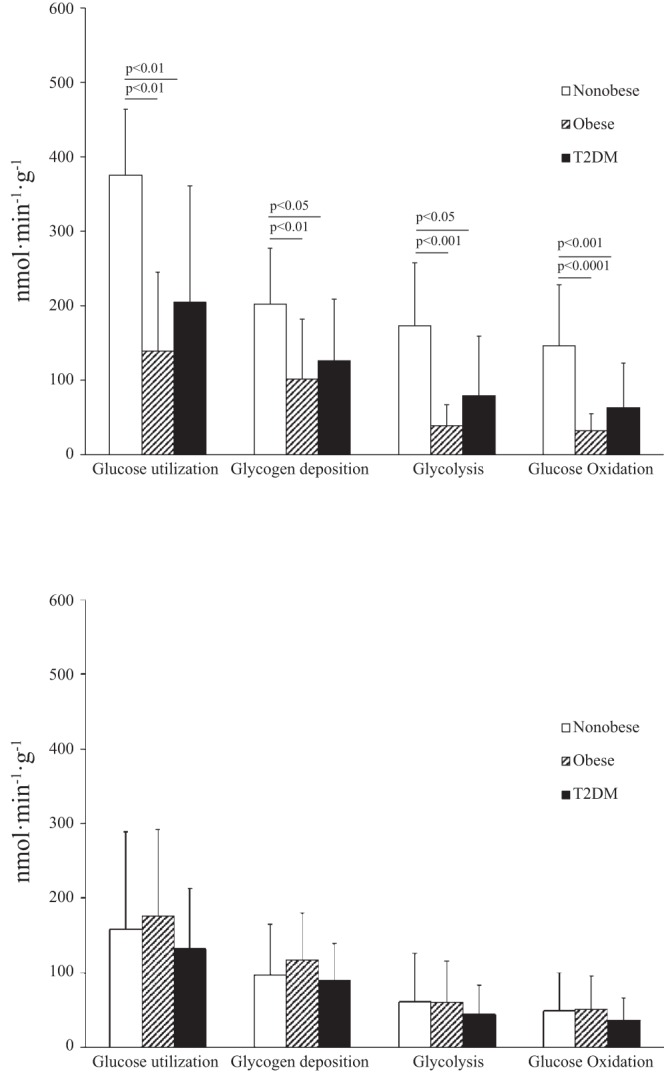
Myocardial glucose utilization and its components in men (top) and women (bottom).
Women.
Glucose utilization did not differ among the three groups of women (Fig. 3, bottom), nor did glycolysis, glycogen deposition, or glucose oxidation rates differ among these groups. The overall blunting of the significance of differences (P values) in glucose utilization compared with fractional glucose uptake among the groups of women was due to higher plasma glucose levels in the T2DM group (Fig. 1).
Multivariate Analyses and Interactions Between Group and Sex Difference in Determining Glucose Uptake and Metabolism
Sex difference and group interacted significantly in the determination of glucose uptake and all its components (Table 3). Group and sex difference were each determinants of fractional myocardial glucose uptake, as well as glycolysis, glycogen, and glucose oxidation deposition (Table 3).
Table 3.
Effects of group, sex difference, and their interaction on myocardial metabolism: results of two-way ANOVA
| Group | Sex Difference | Interaction (Group × Sex Difference) | |
|---|---|---|---|
| Myocardial glucose uptake | 0.0008 | 0.0091 | 0.0016 |
| Glycogen deposition | 0.0024 | 0.0303 | 0.0099 |
| Glycolysis | 0.0008 | 0.0069 | 0.0017 |
| Glucose oxidation | 0.0002 | 0.0040 | 0.0005 |
| Myocardial glucose utilization | 0.0450 | 0.0094 | 0.0173 |
| Glycogen deposition | 0.1941§ | 0.0225 | 0.0586§ |
| Glycolysis | 0.0127 | 0.0096 | 0.0122 |
| Glucose oxidation | 0.0057 | 0.0050 | 0.0050 |
Not statistically significant (P > 0.05).
In the case of glucose utilization, group and sex difference interacted significantly in the determination of glucose utilization and on the metabolic fate of glucose within the cell (Table 3). Group was a determinant of glucose utilization, glycolysis, and glucose oxidation. Sex difference was a determinant of glucose utilization and all its components.
Coupling of Glycolysis With Glucose Oxidation
In the fasted state, the percentage of glucose utilized for glycolysis was relatively low and not statistically different among the groups (Table 4). However, in men and women and in all groups, glycolysis was closely coupled with glucose oxidation (Fig. 4). For example, the portion of glucose that underwent oxidation was ∼83–86% of the glycolytic rate in all groups and both sexes. This would suggest that any differences in the plasma FFA levels are not having a large effect on the glucose metabolism measures (via the Randle cycle), because FFA inhibits glucose oxidation more than glycolysis and glycolysis more than glucose uptake.
Table 4.
Myocardial glycogen deposition vs. glycolysis
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Nonobese | Obese | T2DM | Nonobese | Obese | T2DM | |
| Glycogen deposition | 55 ± 16 | 72 ± 11 | 67 ± 14 | 69 ± 12 | 70 ± 10 | 71 ± 14 |
| Glycolysis | 45 ± 16 | 28 ± 11 | 33 ± 14 | 31 ± 12 | 30 ± 10 | 29 ± 14 |
Values (means ± SD) are expressed as a percentage of glucose utilization.
Fig. 4.
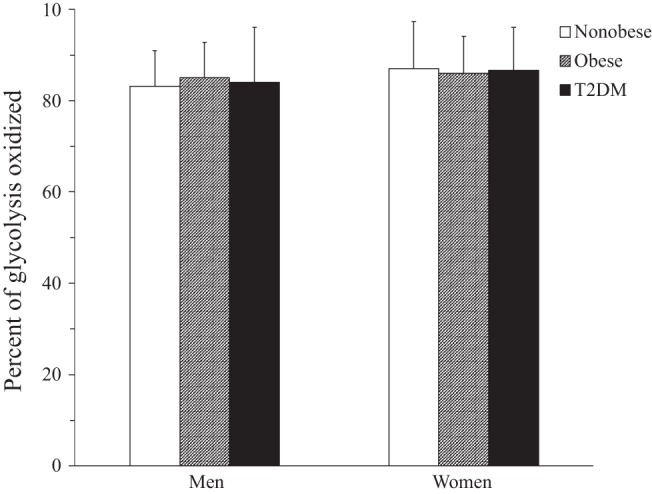
Extracted glucose that undergoes full glucose oxidation shown as percentage of glucose that undergoes glycolysis in men and women. There were no differences among the groups or between men and women.
DISCUSSION
This study is the first to our knowledge to show that T2DM, obesity, and sex difference affect myocardial intracellular glucose metabolism in humans. Knowledge of the intracellular fate of glucose is important, because it determines ATP production (e.g., 1 ATP used for glycogen deposition vs. ∼36 ATP made by full glucose oxidation) for the high-energy demands of the human heart. Determination of the factors that affect the heart's glucose oxidation rate and coupling of glycolysis to glucose oxidation is also vital, because these rates affect the heart's efficiency and adaptability (16). Finally, determination of the point in the chain of human cardiac glucose metabolism at which defects may occur helps identify potential therapeutic targets.
Effects of Diabetes and Obesity on Myocardial Glucose Metabolism
We found that group (T2DM, obese, and nonobese) affected fractional glucose uptake and that the effect on glucose utilization was less pronounced. Results from animal studies suggest that decreased glucose uptake in diabetes may be in part due to decreased GLUT4 and GLUT1 expression or GLUT4 translocation in response to insulin (3). Previous human studies demonstrate that T2DM decreases myocardial glucose uptake in patients (following an oral glucose load) (18) and that obesity and diabetes are associated with decreased fasting cardiac glucose utilization/level of plasma insulin (19, 20). Results of our present study are in agreement with these previous findings, because we found that subject group (T2DM, obese, and nonobese) was related to fractional glucose uptake. Group also was a determinant of glucose utilization.
We also found that diabetes and obesity affected myocardial glycolysis, glucose oxidation, and glycogen deposition rates. Results from animal studies on glycolysis are mixed. Cardiac glycolysis rates are low in ob/ob, but not db/db, mice (4). Another model had higher glycolysis rates (2). We found that group independently predicted the portion of glucose uptake used for glycolysis and that group tended to affect the portion of glucose utilization devoted to glycolysis. Murine studies consistently show that obesity and diabetes decrease oxidation rates (2, 4). These studies further suggest that the decrease in oxidation is not solely due to a decrease in myocardial glucose utilization, but may also be due to a decrease in pyruvate dehydrogenase and/or mitochondrial oxidative capacity (3). We found that group affected the fraction of glucose uptake devoted to glycolysis, glycogen deposition, and glucose oxidation in humans. Group also affected the component parts of glucose utilization: glycolysis, glycogen deposition, and glucose oxidation. However, there was no difference among the groups in terms of the percentage of glycolysis that was needed for glucose oxidation (Fig. 4). In fact, the patterns of glucose oxidation differences among the three groups and in the two sexes mirror the differences in fractional uptake and utilization (Figs. 2 and 3). This suggests that in humans the primary defect in glucose metabolism from diabetes and obesity is in glucose uptake. This decrease in glucose uptake appears to be intrinsic to the myocardium's ability to extract glucose, because blood flow rates are not lower in the obese or the T2DM group. In addition, it suggests that the higher fatty acid levels in the T2DM group did not have a marked effect on glucose metabolism, because fatty acid metabolism typically inhibits glucose oxidation more than glycolysis or glucose uptake (22). Finally, all groups demonstrated robust coupling of glycolysis to glucose oxidation. This also suggests that the amount of potentially damaging protons from uncoupled glycolysis/glucose oxidation in all groups should be low (16).
It is also noteworthy that the significance of the differences between glucose utilization (and its components) in the T2DM group and either of the other two groups is generally less pronounced than the differences in glucose uptake (and its components). In fact, there were no group differences in the portion of glucose utilized for glycogen deposition. This has potential clinical implications. First, it is thought that glycogen may help with cardiac preservation should ischemia supervene (5). The higher plasma glucose levels in patients with diabetes may also enable more glucose utilization by the cell and ameliorate their low glycolysis and glucose oxidation rates. In the ACCORD trial, intensive lowering of blood glucose levels was associated with a worse mortality rate than in patients whose blood glucose was less aggressively managed (7). Whether this outcome difference was due in part to dependence of organs, such as the heart, on higher ambient glucose plasma levels to help maintain intracellular glucose metabolism is an interesting hypothesis requiring further study.
We recognize that many of the adult human's waking hours are not in the fasted state (similar to our testing conditions); however, it is important to evaluate intramyocellular glucose use in the fasting state for several reasons. 1) It is a physiological condition (unlike studies performed during hyperinsulinemic/euglycemic clamp). 2) It is a relatively stable state compared with postprandial conditions. 3) Many important pathophysiological events occur during fasting (e.g., there is a propensity for myocardial infarctions in the early morning, and preoperative patients are usually fasting). However, future study of glucose metabolism under conditions mimicking the postprandial state would likely add additional insights.
Effect of Sex Difference on Intramyocardial Glucose Metabolism
Part of the complexity of evaluating myocardial metabolism in different groups is that sex difference has a profound effect on myocardial metabolism (11, 19–21). In animal models, sex difference affects cardiac triglyceride (1) and FFA metabolism, as well as glucose homeostasis (6). Previously, we showed that, in general, men's hearts have a greater preference for glucose than women's hearts (21). Conversely, women's hearts generally use more fats (20). However, it is only with the development and validation of a new compartmental model (9) that we have been able to determine that sex difference also affected intramyocellular glucose kinetics. In general, the intramyocardial glucose kinetic rates were lower in nonobese and T2DM women than men. The differences in fractional glucose uptake and its components (glycogen synthesis, glycolysis, and glucose oxidation) were generally less pronounced among the groups of women than men. This is likely, in part, because glucose uptake and metabolism rates were relatively low in nonobese women and, therefore, were not markedly different from those in obese and T2DM women. Moreover, glucose utilization (and its components) were not different among the groups of women. This is likely due to relatively low glucose uptake in the nonobese women and higher plasma glucose levels in the T2DM women. Group and sex difference also interacted in the determination of glucose uptake and utilization and all the intracellular metabolism rates. This highlights the complexity of predicting these rates and the need for a more personalized approach to the testing and application of metabolic modulation therapeutics.
Limitations
This study's findings cannot be extrapolated to subjects that do not fit our entry criteria. The subjects were not age-matched across the groups. The T2DM group was older than the other groups. However, aging does not cause a change in resting myocardial glucose utilization (13). Current PET modeling cannot be used to measure endogenous glycogenolysis rates in humans; however, in the resting state, these rates should be exceedingly low (8). The amount of glycogen deposited (as opposed to the rate of deposition) cannot be measured using PET.
Summary
Our current study's results extend our understanding of glucose metabolism in the human heart by demonstrating that group affects the intracellular fate of glucose. Group affected all the components of glucose uptake (glycolysis, glycogen deposition, and glucose oxidation), with the T2DM subjects (both men and women) having low rates. Moreover, the primary defect leading to the decreased intramyocellular glucose metabolism rates appears to be in the myocardium, specifically glucose uptake in humans with T2DM. Sex difference affects myocardial glucose metabolism and interacts with group in its prediction.
Conclusions
T2DM diabetes, obesity, and sex difference have major effects on intracellular glucose handling by the human heart. They often interact in the determination of specific glucose metabolism rates. These factors need to be taken into account in the design and study of metabolic modulator therapy for the heart. Decreased glucose uptake by the human heart appears to be the primary factor limiting glucose oxidation and, hence, a primary target for treatment. Moreover, it appears that a mildly increased plasma glucose level in T2DM patients may help ameliorate the low rates of myocardial glucose metabolism. Thus aggressive lowering of plasma glucose levels (particularly during ischemia, when glucose metabolism is preferred) may be detrimental in patients with diabetes.
GRANTS
This study was funded by National Institutes of Health Grants PO1-HL-013851-43, MO1-RR-000036-461599, P30-DK-056341-08, UL1-RR-024992 (Clinical and Translational Science Award), RO1-HL-073120, and P60-DK-020579-30 and a grant from the Barnes-Jewish Hospital Foundation (St. Louis, MO).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.R.P. and R.J.G. developed the concept and designed the research; L.R.P., I.S., C.D., D.K., J.B.M., M.R.L., and R.J.G. performed the experiments; L.R.P., P.H., Z.K.-W., E.N., V.G.D.-R., A.D.W., and R.J.G. analyzed the data; L.R.P., E.N., and R.J.G. interpreted the results of the experiments; L.R.P. and R.J.G. prepared the figures; L.R.P. and R.J.G. drafted the manuscript; L.R.P., A.R.C., V.G.D.-R., and R.J.G. edited and revised the manuscript; L.R.P. and R.J.G. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Margaret Morton (Washington University School of Medicine) for assistance with manuscript preparation and submission. The authors also thank the subjects who volunteered to participate in the study.
REFERENCES
- 1.Banke NH, Yan L, Pound KM, Dhar S, Reinhardt H, De Lorenzo MS, Vatner SF, Lewandowski ED. Sexual dimorphism in cardiac triacylglyceride dynamics in mice on long term caloric restriction. J Mol Cell Cardiol 52: 733–740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109: 629–639, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 21: 250–258, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341–5349, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 99: 578–588, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor-α-deficient mice. J Clin Invest 102: 1083–1091, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem 34: 29530–29539, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Herrero P, Kisrieva-Ware Z, Dence CS, Patterson B, Coggan AR, Han DH, Ishii Y, Eisenbeis P, Gropler RJ. PET measurements of myocardial glucose metabolism with [1-11C]glucose and kinetic modeling. J Nucl Med 48: 955–964, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Herrero P, Markham J, Bergmann SR. Quantitation of myocardial blood flow with H215O and positron emission tomography: assessment and error analysis of a mathematical approach. J Comput Assist Tomogr 13: 862–873, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Iozzo P, Lautamaki R, Borra R, Lehto HR, Bucci M, Viljanen A, Parkka J, Lepomaki V, Maggio R, Parkkola R, Knuuti J, Nuutila P. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab 94: 4472–4482, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham Study. JAMA 241: 2035–2038, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol 41: 293–299, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 347: 305–313, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation 95: 313–315, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Lyons MR, Peterson LR, McGill JB, Herrero P, Coggan AR, Saeed IM, Recklein C, Schechtman KB, Gropler RJ. Impact of sex on the heart's metabolic and functional responses to diabetic therapies. Am J Physiol Heart Circ Physiol 305: H1584–H1591, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtake T, Yokoyama I, Watanabe T, Momose T, Serezawa T, Nishikawa J, Sasaki Y. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med 36: 456–463, 1995. [PubMed] [Google Scholar]
- 19.Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, Recklein CL, Coggan AR, Demoss AJ, Dence CS, Gropler RJ. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring) 20: 802–810, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, Dence C, Gropler RJ. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging 1: 424–433, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. J Nucl Cardiol 14: 573–581, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes. I. General concepts. Circulation 105: 1727–1733, 2002. [DOI] [PubMed] [Google Scholar]
