Polar Molecule Definition
A polar molecule is a chemical species in which the distribution of electrons between the covalently bonded atoms is not even. Polarity is a description of how different the electrical poles of a molecule are. If they are highly different, it can be said that the species is a highly polar molecule. Some chemical species, such as chains of carbon molecules, share electrons equally and are said to be nonpolar molecules. Typically, the designation of whether a molecule is polar or nonpolar comes from the sum of all of its bonds considered together. Each atom has a certain electronegativity. When bonded to another atom, the atom with the higher electronegativity will tend to attract more electrons. If the difference is not great, a nonpolar bond is formed. If the difference is considerable, a polar bond will form and one atom will attract more electrons. In the more extreme cases, the atom with the greater electronegativity will strip the electrons from the first atom and not share them at all. This creates an ionic bond which is simply an attraction between the two species of atom which are positive and negative. Because they do not share electrons, no physical bond connects these species, and they are considered ions in a matrix and not polar molecules.
Examples of Polar Molecule
Water
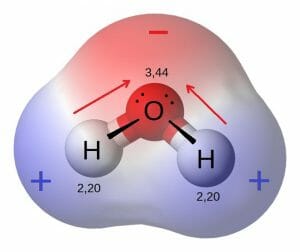
The most important polar molecule on Earth is water. As seen in the image below, water is a polar molecule due to the strong electronegativity of the oxygen atom. This forces most of the electrons to the side of the molecule where oxygen is present, creating a highly negative area. The other side of the molecule becomes more positive, due to the protons of the hydrogen atoms. The polarity of this molecule can create a huge number of reactions in the environment. It can dissolve ions and other polar molecules, and can create temporary hydrogen bonds with other water molecules. Because water is a polar molecule which can interact with other water molecules, it creates a more stable structure. This enables water to have a high heat capacity, or ability to store the energy of heat in these bonds. While it takes a lot of energy to heat water up, it also stays warm for a longer time than most liquids. This is part of the reason life is possible on Earth, because water can carry heat to regions of the plane that receive little sun energy.
Ammonia
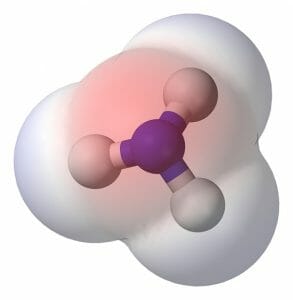
Another simple polar molecule is ammonia. The chemical formula of ammonia is NH3 and it can be seen structurally in the image below. The nitrogen atom, like the oxygen in water, is much more electronegative than the attached hydrogens. This causes an uneven distribution of electrons and makes ammonia a polar molecule. Ammonia, while used as cleaner, is found in nature as a waste product. Fish and other aquatic animals dispel ammonia directly, while terrestrial animal often convert it to urea and other, less-toxic forms. Because ammonia is a polar molecule, it can be dissolved by water. As ammonia is a by-product of the breakdown and creation of proteins and bodily substances, most excretory systems rely on water to flush ammonia from the body.
Related Biology Terms
- Polarity – The measure of electrical difference within a molecule, bond, or structure.
- Nonpolar Molecule – A molecules make of electronegatively similar atoms, which distributes electrons equally.
- Amphiphilic Molecule – Some large molecules which have both polar and nonpolar regions, such as the phospholipids used to create cell membranes.
- Electronegativity – The measure of the attraction an atom has for electrons, which can determine the polarity of bonds formed with that atom.
Quiz
1. When sodium (Na) and chlorine (Cl) combine, the highly electronegative chlorine steals all the electrons from sodium. The two then exist in a matrix, the highly positive Na+ ions being attracted to the negative Cl– ions. Which type of molecule is this?
A. Polar Molecule
B. Nonpolar Molecule
C. Not a molecule
2. The following elements have the corresponding electronegativity:
Oxygen: 3.44
Chlorine: 3.16
Hydrogen: 2.2
Which of the following substances is the MOST polar molecule?
A. HCl
B. H2O
C. O2
3. As discussed in the article, ammonia is a polar molecule produced as a waste product. Why do fish continue to produce ammonia, while terrestrial organisms have switched to less toxic forms of waste?
A. Fish are less evolved than terrestrial organisms.
B. Terrestrial organisms needed a nonpolar molecule.
C. Terrestrial organisms have a need to conserve water.