Norrish reaction
A Norrish reaction, named after Ronald George Wreyford Norrish, is a photochemical reaction taking place with ketones and aldehydes. Such reactions are subdivided into Norrish type I reactions and Norrish type II reactions.[1] While of limited synthetic utility these reactions are important in the photo-oxidation of polymers such as polyolefins,[2] polyesters, certain polycarbonates and polyketones.
Type I
[edit]The Norrish type I reaction is the photochemical cleavage or homolysis of aldehydes and ketones into two free radical intermediates (α-scission). The carbonyl group accepts a photon and is excited to a photochemical singlet state. Through intersystem crossing the triplet state can be obtained. On cleavage of the α-carbon bond from either state, two radical fragments are obtained.[3] The size and nature of these fragments depends upon the stability of the generated radicals; for instance, the cleavage of 2-butanone largely yields ethyl radicals in favor of less stable methyl radicals.[4]
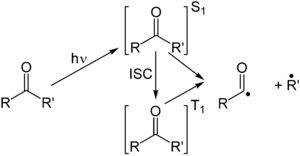
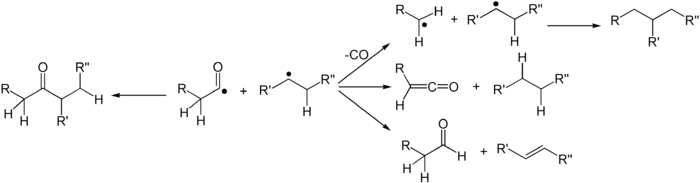
Several secondary reaction modes are open to these fragments depending on the exact molecular structure.
- The fragments can simply recombine to the original carbonyl compound, with racemisation at the α-carbon.
- The acyl radical can lose a molecule of carbon monoxide, forming a new carbon radical at the other α-carbon, followed by formation of a new carbon–carbon bond between the radicals.[3] The ultimate effect is simple extraction of the carbonyl unit from the carbon chain. The rate and yield of this product depends upon the bond-dissociation energy of the ketone's α substituents. Typically the more α substituted a ketone is, the more likely the reaction will yield products in this way.[5][6]
- The abstraction of an α-proton from the carbonyl fragment may form a ketene and an alkane.
- The abstraction of a β-proton from the alkyl fragment may form an aldehyde and an alkene.
The synthetic utility of this reaction type is limited, for instance it often is a side reaction in the Paternò–Büchi reaction. One organic synthesis based on this reaction is that of bicyclohexylidene.[7] The Norrish Type I reaction plays a crucial role in the field of photopolymerization, particularly in the development of photoinitiators used for two-photon polymerization (2PP). The Norrish Type I reaction is particularly significant here because it involves the cleavage of a carbon-carbon bond in a photoinitiator molecule upon excitation by UV or visible light, leading to the formation of two radical species. These radicals are highly reactive and can effectively initiate the polymerization of monomers in a localized region, allowing for the precise 3D structuring required in two-photon polymerization processes. This makes the Norrish Type I reaction a fundamental mechanism for designing photoinitiators that are capable of driving high-resolution additive manufacturing at the microscale.[8]
Type II
[edit]A Norrish type II reaction is the photochemical intramolecular abstraction of a γ-hydrogen (a hydrogen atom three carbon positions removed from the carbonyl group) by the excited carbonyl compound to produce a 1,4-biradical as a primary photoproduct.[9] Norrish first reported the reaction in 1937.[10]
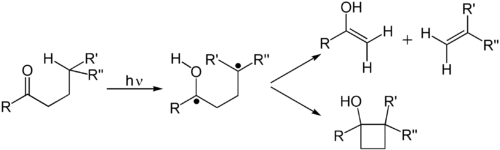
Secondary reactions that occur are fragmentation (β-scission) to form an alkene and an enol (which will rapidly tautomerise to a carbonyl), or intramolecular recombination of the two radicals to a substituted cyclobutane (the Norrish–Yang reaction).[11]
Scope
[edit]The Norrish reaction has been studied in relation to environmental chemistry with respect to the photolysis of the aldehyde heptanal, a prominent compound in Earth's atmosphere.[12] Photolysis of heptanal in conditions resembling atmospheric conditions results in the formation of 1-pentene and acetaldehyde in 62% chemical yield together with cyclic alcohols (cyclobutanols and cyclopentanols) both from a Norrish type II channel and around 10% yield of hexanal from a Norrish type I channel (the initially formed n-hexyl radical attacked by oxygen).
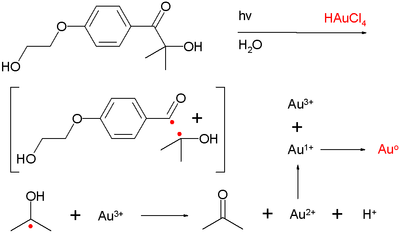
In one study[13] the photolysis of an acyloin derivative in water in presence of hydrogen tetrachloroaurate (HAuCl4) generated nanogold particles with 10 nanometer diameter. The species believed to responsible for reducing Au3+ to Au0[14] is the Norrish generated ketyl radical.
Leo Paquette's 1982 synthesis of dodecahedrane involves three separate Norrish-type reactions in its approximately 29-step sequence.
An example of a synthetically useful Norrish type II reaction can be found early in the total synthesis of the biologically active cardenolide ouabagenin by Phil Baran and coworkers.[15] The optimized conditions minimize side reactions, such as the competing Norrish type I pathway, and furnish the desired intermediate in good yield on a multi-gram scale.

See also
[edit]- Photo-Fries rearrangement - a related reaction of aromatic carbonyls
- McLafferty rearrangement - similar to a Type II Norrish reaction. Caused by electron impact ionization rather than light
- Carbon monoxide-releasing molecules
References
[edit]- ^ Named Organic Reactions, 2nd Edition, Thomas Laue and Andreas Plagens, John Wiley & Sons: Chichester, England, New York, 2005. 320 pp. ISBN 0-470-01041-X
- ^ Grause, Guido; Chien, Mei-Fang; Inoue, Chihiro (November 2020). "Changes during the weathering of polyolefins". Polymer Degradation and Stability. 181: 109364. doi:10.1016/j.polymdegradstab.2020.109364. S2CID 225243217.
- ^ a b "IUPAC Gold Book - Norrish Type I photoreaction". IUPAC. 24 February 2014. doi:10.1351/goldbook.N04219. Retrieved 31 March 2014.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Blacet, F. E.; N. Pitts Jr., James (1950). "Methyl Ethyl Ketone Photochemical Processes". Journal of the American Chemical Society. 72 (6): 2810–2811. doi:10.1021/ja01162a544.
- ^ Yang, Nien-Chu; D. Feit, Eugene; Hui, Man Him; Turro, Nicholas J.; Dalton, Christopher (1970). "Photochemistry of di-tert-butyl ketone and structural effects on the rate and efficiency of intersystem crossing of aliphatic ketones". Journal of the American Chemical Society. 92 (23): 6974–6976. doi:10.1021/ja00726a046.
- ^ Abuin, E.B.; Encina, M.V.; Lissi, E.A. (1972). "The photolysis of 3-pentanone". Journal of Photochemistry. 1 (5): 387–396. doi:10.1016/0047-2670(72)80036-4.
- ^ Bicyclohexylidene Nicholas J. Turro, Peter A. Leermakers, and George F. Vesley Organic Syntheses, Coll. Vol. 5, p.297 (1973); Vol. 47, p.34 (1967) Online article.
- ^ Ushiba, Shota; Masui, Kyoko; Taguchi, Natsuo; Hamano, Tomoki; Kawata, Satoshi; Shoji, Satoru (2015-11-27). "Size dependent nanomechanics of coil spring shaped polymer nanowires". Scientific Reports. 5 (1): 17152. doi:10.1038/srep17152. ISSN 2045-2322. PMC 4661696. PMID 26612544.
- ^ "IUPAC Gold Book - Norrish Type II photoreaction". IUPAC. 24 February 2014. doi:10.1351/goldbook.N04218. Retrieved 31 March 2014.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Norrish, R. G. W.; Bamford, C. H. (31 July 1937). "Photo-decomposition of Aldehydes and Ketones". Nature. 140 (3535): 195–6. Bibcode:1937Natur.140..195N. doi:10.1038/140195b0. S2CID 4104669.
- ^ "IUPAC Gold Book - Norrish–Yang reaction". IUPAC. 24 February 2014. doi:10.1351/goldbook.NT07427. Retrieved 31 March 2014.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Photolysis of Heptanal Suzanne E. Paulson, De-Ling Liu, Grazyna E. Orzechowska, Luis M. Campos, and K. N. Houk J. Org. Chem.; 2006; 71(17) pp 6403 - 6408; (Article) doi:10.1021/jo060596u
- ^ Facile Photochemical Synthesis of Unprotected Aqueous Gold Nanoparticles Katherine L. McGilvray, Matthew R. Decan, Dashan Wang, and Juan C. Scaiano J. Am. Chem. Soc.; 2006; 128(50) pp 15980 - 15981; (Communication) doi:10.1021/ja066522h
- ^ Technically Au3+ is reduced to Au2+ which then forms Au+ and Au3+ by disproportionation followed by final reduction of Au1+ to Auo
- ^ Renata, H.; Zhou, Q.; Baran, P. S. (3 January 2013). "Strategic Redox Relay Enables A Scalable Synthesis of Ouabagenin, A Bioactive Cardenolide". Science. 339 (6115): 59–63. Bibcode:2013Sci...339...59R. doi:10.1126/science.1230631. PMC 4365795. PMID 23288535.