Capsaicin
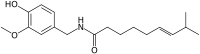 |
|
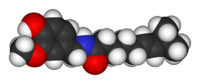 |
|
| Names | |
|---|---|
| IUPAC name
(E)-N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide
|
|
| Other names
8-Methyl-N-vanillyl-trans-6-nonenamide; trans-8-Methyl-N-vanillylnon-6-enamide; (E)-Capsaicin; Capsicine; Capsicin; CPS
|
|
| Identifiers | |
| 404-86-4 |
|
| ChEBI | CHEBI:3374 |
| ChEMBL | ChEMBL294199 |
| ChemSpider | 1265957 |
| EC Number | 206-969-8 |
| 2486 | |
| Jmol 3D model | Interactive image |
| KEGG | C06866 |
| PubChem | 1548943 |
| UNII | S07O44R1ZM |
|
|
|
|
| Properties | |
| C18H27NO3 | |
| Molar mass | 305.42 g·mol−1 |
| Appearance | crystalline white powder[1] |
| Odor | highly volatile and pungent |
| Melting point | 62 to 65 °C (144 to 149 °F; 335 to 338 K) |
| Boiling point | 210 to 220 °C (410 to 428 °F; 483 to 493 K) 0.01 Torr |
| 0.0013 g/100 mL | |
| Solubility | soluble in alcohol, ether, benzene slightly soluble in CS2, HCl, petroleum |
| UV-vis (λmax) | 280 nm |
| Structure | |
| monoclinic | |
| Pharmacology | |
| ATC code | M02 N01BX04 |
| Vapor pressure | {{{value}}} |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
| Capsaicin | |
|---|---|
| Heat | Above Peak (SR: 15,000,000-16,000,000) |
Capsaicin (/kæpˈseɪ.ᵻsɪn/ (INN); 8-methyl-N-vanillyl-6-nonenamide) is an active component of chili peppers, which are plants belonging to the genus Capsicum. It is an irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related compounds are called capsaicinoids and are produced as secondary metabolites by chili peppers, probably as deterrents against certain mammals and fungi.[2] Pure capsaicin is a volatile, hydrophobic, colorless, odorless, crystalline to waxy compound.
Contents
History
The compound was first extracted in impure form in 1816 by Christian Friedrich Bucholz (1770–1818).[3] He called it "capsicin", after the genus Capsicum from which it was extracted. John Clough Thresh (1850–1932), who had isolated capsaicin in almost pure form,[4][5] gave it the name "capsaicin" in 1876.[6] Karl Micko isolated capsaicin in its pure form in 1898.[7] Capsaicin's chemical composition was first determined by E. K. Nelson in 1919, who also partially elucidated capsaicin's chemical structure.[8] Capsaicin was first synthesized in 1930 by E. Spath and S. F. Darling.[9] In 1961, similar substances were isolated from chili peppers by the Japanese chemists S. Kosuge and Y. Inagaki, who named them capsaicinoids.[10][11]
In 1873 German pharmacologist Rudolf Buchheim[12] (1820–1879) and in 1878 the Hungarian doctor Endre Hőgyes[13] stated that "capsicol" (partially purified capsaicin[14]) caused the burning feeling when in contact with mucous membranes and increased secretion of gastric acid.
Capsaicinoids
Capsaicin is the main capsaicinoid in chili peppers, followed by dihydrocapsaicin. These two compounds are also about twice as potent to the taste and nerves as the minor capsaicinoids nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin. Dilute solutions of pure capsaicinoids produced different types of pungency; however, these differences were not noted using more concentrated solutions.
Capsaicin is believed to be synthesized in the interlocular septum of chili peppers by addition of a branched-chain fatty acid to vanillylamine; specifically, capsaicin is made from vanillylamine and 8-methyl-6-nonenoyl CoA.[15][16] Biosynthesis depends on the gene AT3, which resides at the pun1 locus, and which encodes a putative acyltransferase.[17]
Besides the six natural capsaicinoids, one synthetic member of the capsaicinoid family exists. Vanillylamide of n-nonanoic acid (VNA, also PAVA) is used as a reference substance for determining the relative pungency of capsaicinoids.
| Capsaicinoid name | Abbrev. | Typical relative amount |
Scoville heat units |
Chemical structure |
|---|---|---|---|---|
| Capsaicin | C | 69% | 16,000,000 |  |
| Dihydrocapsaicin | DHC | 22% | 15,000,000 | 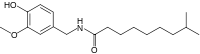 |
| Nordihydrocapsaicin | NDHC | 7% | 9,100,000 | 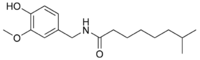 |
| Homodihydrocapsaicin | HDHC | 1% | 8,600,000 | 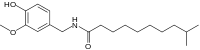 |
| Homocapsaicin | HC | 1% | 8,600,000 | 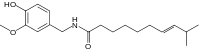 |
| Nonivamide | PAVA | 9,200,000 | 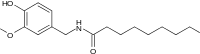 |
Natural function
Capsaicin is present in large quantities in the placental tissue (which holds the seeds), the internal membranes and, to a lesser extent, the other fleshy parts of the fruits of plants in the genus Capsicum. The seeds themselves do not produce any capsaicin, although the highest concentration of capsaicin can be found in the white pith of the inner wall, where the seeds are attached.[18]
The seeds of Capsicum plants are dispersed predominantly by birds: in birds, the TRPV1 channel does not respond to capsaicin or related chemicals (avian vs mammalian TRPV1 show functional diversity and selective sensitivity). This is advantageous to the plant, as chili pepper seeds consumed by birds pass through the digestive tract and can germinate later, whereas mammals have molar teeth which destroy such seeds and prevent them from germinating. Thus, natural selection may have led to increasing capsaicin production because it makes the plant less likely to be eaten by animals that do not help it disperse.[19] There is also evidence that capsaicin may have evolved as an anti-fungal agent:[20] the fungal pathogen Fusarium, which is known to infect wild chilies and thereby reduce seed viability, is deterred by capsaicin, which thus limits this form of predispersal seed mortality.
In 2006, it was discovered that the venom of a certain tarantula species activates the same pathway of pain as is activated by capsaicin; this was the first demonstrated case of such a shared pathway in both plant and animal anti-mammal defense.[21]
Uses
Food
Because of the burning sensation caused by capsaicin when it comes in contact with mucous membranes, it is commonly used in food products to provide added spice or "heat" (piquancy).[22] In high concentrations, capsaicin will also cause a burning effect on other sensitive areas, such as skin or eyes.[23] The degree of heat found within a food is often measured on the Scoville scale.[22] In some cases, because people enjoy the heat,[22] there has long been a demand for capsaicin-spiced products like hot sauce or salsa.[22]
It is common for people to experience pleasurable and even euphoriant effects from ingesting capsaicin.[22] Folklore among self-described "chiliheads" attributes this to pain-stimulated release of endorphins, a different mechanism from the local receptor overload that makes capsaicin effective as a topical analgesic.[23]
Research and pharmaceutical use
Capsaicin is used as an analgesic in topical ointments, nasal sprays (Sinol-M), and dermal patches to relieve pain, typically in concentrations between 0.025% and 0.25%. It may be applied in cream form for the temporary relief of minor aches and pains of muscles and joints associated with arthritis, backache, strains and sprains, often in compounds with other rubefacients.[24] It is also used to reduce the symptoms of peripheral neuropathy such as post-herpetic neuralgia caused by shingles.[25] Capsaicin transdermal patch (Qutenza) for the management of this particular therapeutic indication (pain due to post-herpetic neuralgia) was approved as a therapeutic by the U.S. FDA,[26] but a subsequent application for Qutenza to be used as an analgesic in HIV neuralgia was refused by the FDA.[27]
Although capsaicin creams have been used to treat psoriasis for reduction of itching,[28][29] a review of six clinical trials involving topical capsaicin for treatment of pruritus concluded there was insufficient evidence of effect.[30]
The American Cancer Society warns "available scientific research does not support claims for the effectiveness of capsicum or whole pepper supplements in preventing or curing cancer at this time".[31] Other uses not supported by evidence are: "addiction, malaria, yellow fever, heart disease, stroke, weight loss, poor appetite, and sexual potency".[31]
One observational prospective study of 487,375 people over 7.2 years showed an inverse correlation between spicy food consumption and mortality resulting from cancer, ischemic heart disease or respiratory diseases, but the study was not designed to discern causes of this relationship.[32]
Pepper spray and pests
Capsaicin is also an active ingredient in riot control and personal defense pepper spray agents.[citation needed] When the spray comes in contact with skin, especially eyes or mucous membranes, it produces pain and breathing difficulty, discouraging assailants. Refer to the Scoville scale for a comparison of pepper spray to other sources of capsaicin.
Capsaicin is also used to deter pests, specifically mammalian pests. Targets of capsaicin repellants include voles, deer, rabbits, squirrels, insects, and attacking dogs.[33] Ground or crushed dried chili pods may be used in birdseed to deter rodents,[34] taking advantage of the insensitivity of birds to capsaicin. The Elephant Pepper Development Trust claims the use of chili peppers to improve crop security for rural African communities[citation needed]. Notably, an article published in the Journal of Environmental Science and Health in 2006 states that "Although hot chili pepper extract is commonly used as a component of household and garden insect-repellent formulas, it is not clear that the capsaicinoid elements of the extract are responsible for its repellency."[35]
The first pesticide product using solely capsaicin as the active ingredient was registered with the U.S. Department of Agriculture in 1962.[33]
Equestrian sports
Capsaicin is a banned substance in equestrian sports because of its hypersensitizing and pain-relieving properties. At the show jumping events of the 2008 Summer Olympics, four horses tested positive for the substance, which resulted in disqualification.[36]
Mechanism of action
The burning and painful sensations associated with capsaicin result from its chemical interaction with sensory neurons. Capsaicin, as a member of the vanilloid family, binds to a receptor called the vanilloid receptor subtype 1 (TRPV1).[37] First cloned in 1997, TRPV1 is an ion channel-type receptor.[38] TRPV1, which can also be stimulated with heat, protons and physical abrasion, permits cations to pass through the cell membrane when activated. The resulting depolarization of the neuron stimulates it to signal the brain. By binding to the TRPV1 receptor, the capsaicin molecule produces similar sensations to those of excessive heat or abrasive damage, explaining why the spiciness of capsaicin is described as a burning sensation.
Early research showed capsaicin to evoke a strikingly long-onset current in comparison to other chemical agonists, suggesting the involvement of a significant rate-limiting factor.[39] Subsequent to this, the TRPV1 ion channel has been shown to be a member of the superfamily of TRP ion channels, and as such is now referred to as TRPV1. There are a number of different TRP ion channels that have been shown to be sensitive to different ranges of temperature and probably are responsible for our range of temperature sensation. Thus, capsaicin does not actually cause a chemical burn, or indeed any direct tissue damage at all, when chili peppers are the source of exposure. The inflammation resulting from exposure to capsaicin is believed to be the result of the body's reaction to nerve excitement. For example, the mode of action of capsaicin in inducing bronchoconstriction is thought to involve stimulation of C fibers[40] culminating in the release of neuropeptides. In essence, the body inflames tissues as if it has undergone a burn or abrasion and the resulting inflammation can cause tissue damage in cases of extreme exposure, as is the case for many substances that cause the body to trigger an inflammatory response.
Toxicity
Acute health effects
Capsaicin is a highly irritant material requiring proper protective goggles, respirators, and proper hazardous material-handling procedures. Capsaicin takes effect upon skin contact (irritant, sensitizer), eye contact (irritant), ingestion, and inhalation (lung irritant, lung sensitizer). The LD50 in mice is 47.2 mg/kg.[41][42]
Painful exposures to capsaicin-containing peppers are among the most common plant-related exposures presented to poison centers.[citation needed] They cause burning or stinging pain to the skin and, if ingested in large amounts by adults or small amounts by children, can produce nausea, vomiting, abdominal pain, and burning diarrhea. Eye exposure produces intense tearing, pain, conjunctivitis, and blepharospasm.[43]
When used for weight loss in capsules, there has been a report of heart attack; this was thought to be due to excess sympathetic output.[44]
Treatment after exposure
The primary treatment is removal from exposure. Contaminated clothing should be removed and placed in airtight bags to prevent secondary exposure.
For external exposure, bathing the mucous membrane surfaces that have contacted capsaicin with oily compounds such as vegetable oil, paraffin oil, petroleum jelly (Vaseline), creams, or polyethylene glycol is the most effective way to attenuate the associated discomfort;[citation needed] since oil and capsaicin are both hydrophobic hydrocarbons the capsaicin that has not already been absorbed into tissues will be picked up into solution and easily removed. Capsaicin can also be washed off the skin using soap, shampoo, or other detergents. Plain water is ineffective at removing capsaicin,[41] as are bleach, sodium metabisulfite and topical antacid suspensions.[citation needed] Capsaicin is soluble in alcohol, which can be used to clean contaminated items.[41]
When capsaicin is ingested, cold milk is an effective way to relieve the burning sensation (due to caseins having a detergent effect on capsaicin[45]); and room-temperature sugar solution (10%) at 20 °C (68 °F) is almost as effective.[46] The burning sensation will slowly fade away over several hours if no actions are taken.
Capsaicin-induced asthma might be treated with oral antihistamines or corticosteroids.[43]
Effects of dietary consumption
Ingestion of spicy food or ground jalapeño peppers does not cause mucosal erosions or other abnormalities.[47] Some mucosal microbleeding has been found after eating red and black peppers, but there was no significant difference between aspirin (used as a control) and peppers.[48] The question of whether chili ingestion increases or decreases risk of stomach cancer is mixed: a study of Mexican patients found self-reported capsaicin intake levels associated with increased stomach cancer rates (and this is independent of infection with Helicobacter pylori[49]) while a study of Italians suggests eating hot peppers regularly was protective against stomach cancer.[50] Carcinogenic, co-carcinogenic, and anticarcinogenic effects of capsaicin have been reported in animal studies.[51][52]
Effects on weight loss and regain
There is no evidence showing that weight loss is directly correlated with ingesting capsaicin, but there is preliminary evidence for capsaicin possibly affecting weight regain where capsaicin may produce "a shift in substrate oxidation from carbohydrate to fat oxidation".[53] The effect may elicit a decrease in appetite as well as a decrease in food intake.[53] In one study, oral and gastrointestinal exposure to capsaicin increased satiety and reduced food intake.[54] Long-term studies are limited because of the pungency of capsaicin.[55]
See also
- Allicin, the active piquant flavor chemical in uncooked garlic, and to a lesser extent onions (see those articles for discussion of other chemicals in them relating to pungency, and eye irritation)
- Allyl isothiocyanate, the active piquant chemical in mustard, radishes, horseradish, and wasabi
- Capsazepine, capsaicin antagonist
- Capsinoids, similar in structure to capsaicin, but lack the extreme pungency, and density
- Discovery and development of TRPV1 antagonists
- Gingerol and shogaol, the active piquant flavor chemicals in ginger
- Naga Viper pepper, Bhut Jolokia Pepper, Carolina Reaper, Trinidad Moruga Scorpion; some of the world's most capsaicin-rich fruits
- Pepper spray, used in policing, riot control, crowd control, and personal self-defense, including defense against dogs and bears
- Piperine, the active piquant chemical in black pepper
- Resiniferatoxin, an ultrapotent capsaicin analog in Euphorbia plants
- Scoville scale, a measurement of the spicy heat (or pungency) of a chili pepper
- syn-Propanethial-S-oxide, the major active piquant chemical in onions
- TRPV1, the only known receptor (a transient receptor potential channel) for capsaicin
References
Footnotes
- ↑ ChemSpider - Capsaicin
- ↑ What Made Chili Peppers So Spicy? Talk of the Nation, 15 August 2008.
- ↑ History of early research on capsaicin:
- Harvey W. Felter and John U. Lloyd, King's American Dispensatory (Cincinnati, Ohio: Ohio Valley Co., 1898), vol. 1, page 435. Available on-line at: Henriette's Herbal.
- Andrew G. Du Mez, "A century of the United States pharmocopoeia 1820-1920. I. The galenical oleoresins" (Ph.D. dissertation, University of Wisconsin, 1917), pages 111-132. Available on-line at: Archive.org.
- C. F. Bucholz (1816) "Chemische Untersuchung der trockenen reifen spanischen Pfeffers" [Chemical investigation of dry, ripe Spanish peppers], Almanach oder Taschenbuch für Scheidekünstler und Apotheker (Weimar) [Almanac or Pocket-book for Analysts (Chemists) and Apothecaries], vol. 37, pages 1-30. [Note: Christian Friedrich Bucholz's surname has been variously spelled as "Bucholz", "Bucholtz", or "Buchholz".]
- The results of Bucholz's and Braconnot's analyses of Capsicum annuum appear in: Jonathan Pereira, The Elements of Materia Medica and Therapeutics, 3rd U.S. ed. (Philadelphia, Pennsylvania: Blanchard and Lea, 1854), vol. 2, page 506.
- Biographical information about Christian Friedrich Bucholz is available in: Hugh J. Rose, Henry J. Rose, and Thomas Wright, ed.s, A New General Biographical Dictionary (London, England: 1857), vol. 5, page 186.
- Biographical information about C. F. Bucholz is also available (in German) on-line at: Allgemeine Deutsche Biographie.
- Some other early investigators who also extracted the active component of peppers:
-
- Benjamin Maurach (1816) "Pharmaceutisch-chemische Untersuchung des spanischen Pfeffers" (Pharmaceutical-chemical investigation of Spanish peppers), Berlinisches Jahrbuch für die Pharmacie, vol. 17, pages 63-73. Abstracts of Maurach's paper appear in: (i) Repertorium für die Pharmacie, vol. 6, page 117-119 (1819); (ii) Allgemeine Literatur-Zeitung, vol. 4, no. 18, page 146 (Feb. 1821); (iii) "Spanischer oder indischer Pfeffer", System der Materia medica ... , vol. 6, pages 381-386 (1821) (this reference also contains an abstract of Bucholz's analysis of peppers).
- French chemist Henri Braconnot (1817) "Examen chemique du Piment, de son principe âcre, et de celui des plantes de la famille des renonculacées" (Chemical investigation of the chili pepper, of its pungent principle [constituent, component], and of that of plants of the family Ranunculus), Annales de Chemie et de Physique, vol. 6, pages 122- 131.
- Danish geologist Johann Georg Forchhammer in: Hans C. Oersted (1820) "Sur la découverte de deux nouveaux alcalis végétaux" (On the discovery of two new plant alkalis), Journal de physique, de chemie, d'histoire naturelle et des arts, vol. 90, pages 173-174.
- German apothecary Ernst Witting (1822) "Considerations sur les bases vegetales en general, sous le point de vue pharmaceutique et descriptif de deux substances, la capsicine et la nicotianine" (Thoughts on the plant bases in general from a pharmaceutical viewpoint, and description of two substances, capsicin and nicotine), Beiträge für die pharmaceutische und analytische Chemie, vol. 3, pages 43ff.
- ↑ In a series of articles, J. C. Thresh obtained capsaicin in almost pure form:
- J. C. Thresh (1876) "Isolation of capsaicin," The Pharmaceutical Journal and Transactions, 3rd series, vol. 6, pages 941-947;
- J. C. Thresh (8 July 1876) "Capsaicin, the active principle in Capsicum fruits," The Pharmaceutical Journal and Transactions, 3rd series, vol. 7, no. 315, pages 21 ff. [Note: This article is summarized in: "Capsaicin, the active principle in Capsicum fruits," The Analyst, vol. 1, no. 8, pages 148-149, (1876).]. In The Pharmaceutical Journal and Transactions, volume 7, see also pages 259ff and 473 ff and in vol. 8, see pages 187ff;
- Year Book of Pharmacy… (1876), pages 250 and 543;
- J. C. Thresh (1877) "Note on Capsaicin," Year Book of Pharmacy…, pages 24-25;
- J. C. Thresh (1877) "Report on the active principle of Cayenne pepper," Year Book of Pharmacy..., pages 485-488.
- ↑ Obituary notice of J. C. Thresh: "John Clough Thresh, M.D., D. Sc., and D.P.H.," The British Medical Journal, vol. 1, no. 3726, pages 1057-1058 (4 June 1932).
- ↑ J King, H Wickes Felter, J Uri Lloyd (1905) A King's American Dispensatory. Eclectic Medical Publications (ISBN 1888483024)
- ↑ Karl Micko (1898) "Zur Kenntniss des Capsaïcins" (On our knowledge of capsaicin), Zeitschrift für Untersuchung der Nahrungs- und Genussmittel (Journal for the Investigation of Necessities and Luxuries), vol. 1, pages 818-829. See also: Karl Micko (1899) "Über den wirksamen Bestandtheil des Cayennespfeffers" (On the active component of Cayenne pepper), Zeitschrift für Untersuchung der Nahrungs- und Genussmittel, vol. 2, pages 411-412.
- ↑ E. K. Nelson. "The constitution of capsaicin, the pungent principle of capsicum". J. Am. Chem. Soc. 1919; 41: 1115–1121. doi 10.1021/ja02228a011
- ↑ Ernst Späth, Stephen F. Darling. "Synthese des Capsaicins." Chem. Ber. 1930; 63B: 737–743.
- ↑ S Kosuge, Y Inagaki, H Okumura (1961). Studies on the pungent principles of red pepper. Part VIII. On the chemical constitutions of the pungent principles. Nippon Nogei Kagaku Kaishi (J. Agric. Chem. Soc.), 35, 923–927; (en) Chem. Abstr. 1964, 60, 9827g.
- ↑ (ja) S Kosuge, Y Inagaki (1962) Studies on the pungent principles of red pepper. Part XI. Determination and contents of the two pungent principles. Nippon Nogei Kagaku Kaishi J. Agric. Chem. Soc., 36, pp. 251
- ↑ Rudolf Buchheim (1873) "Über die 'scharfen' Stoffe" (On the "hot" substance), Archiv der Heilkunde (Archive of Medicine), vol. 14, pages 1ff. See also: R. Buchheim (1872) "Fructus Capsici," Vierteljahresschrift fur praktische Pharmazie (Quarterly Journal for Practical Pharmacy), vol. 4, pages 507ff.; reprinted (in English) in: Proceedings of the American Pharmaceutical Association, vol. 22, pages 106ff (1873).
- ↑ Endre Hőgyes, "Adatok a paprika (Capsicum annuum) élettani hatásához" [Data on the physiological effects of the pepper (Capsicum annuum)], Orvos-természettudumányi társulatot Értesítője [Bulletin of the Medical Science Association] (1877); reprinted in: Orvosi Hetilap [Medical Journal] (1878), 10 pages. Published in German as: "Beitrage zur physiologischen Wirkung der Bestandtheile des Capiscum annuum (Spanischer Pfeffer)" [Contributions on the physiological effects of components of Capsicum annuum (Spanish pepper)], Archiv für Experimentelle Pathologie und Pharmakologie, vol. 9, pages 117-130 (1878). See springerlink.com
- ↑ F.A. Flückiger, Pharmakognosie des Pflanzenreiches ( Berlin, Germany: Gaertner's Verlagsbuchhandlung, 1891).
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ I. Guzman, P.W. Bosland, and M.A. O'Connell, "Chapter 8: Heat, Color, and Flavor Compounds in Capsicum Fruit" in David R. Gang, ed., Recent Advances in Phytochemistry 41: The Biological Activity of Phytochemicals (New York, New York: Springer, 2011), pages 117-118.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 22.0 22.1 22.2 22.3 22.4 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 23.0 23.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Topical capsaicin for pain relief
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 31.0 31.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 33.0 33.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Geppetti, Pierangelo & Holzer, Peter (1996). Neurogenic Inflammation. CRC Press, 1996.
- ↑ Fuller, R. W., Dixon, C. M. S. & Barnes, P. J. (1985). Bronchoconstrictor response to inhaled capsaicin in humans" J. Appl. Physiol 58, 1080–1084. PubMed, CAS, Web of Science® Times Cited: 174
- ↑ 41.0 41.1 41.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 43.0 43.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Sayin MR, et al. A case of acute myocardial infarction due to the use of cayenne pepper pills. Wiener Klinische Wochenschrift-The Central European Journal of Medicine (2012) 124:285-287
- ↑ General Chemistry Online: Fire and Spice
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 53.0 53.1 Lejeune, Manuela P. G. M., Eva M. R. Kovacs, and Margriet S. Westerterp- Plantenga. "Effect of Capsaicin on Substrate Oxidation and Weight Maintenance after Modest Body-weight Loss in Human Subjects." British Journal of Nutrition 90.03 (2003): 651.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Diepvens, K., K. R. Westerterp, and M. S. Westerterp-Plantenga. "Obesity and Thermogenesis Related to the Consumption of Caffeine, Ephedrine, Capsaicin, and Green Tea." AJP: Regulatory, Integrative and Comparative Physiology 292.1 (2006): R77-85.
General references
- Lua error in package.lua at line 80: module 'strict' not found.
- Garnanez RJ, McKee LH (2001) "Temporal effectiveness of sugar solutions on mouth burn by capsaicin" IFT Annual Meeting 2001
- Lua error in package.lua at line 80: module 'strict' not found.
- Lua error in package.lua at line 80: module 'strict' not found.
- Lua error in package.lua at line 80: module 'strict' not found.
- Lua error in package.lua at line 80: module 'strict' not found.
- Tarantula Venom, Chili Peppers Have Same "Bite", Study Finds http://news.nationalgeographic.com/news/2006/11/061108-tarantula-venom.html
- Minna M. Hamalainen, Alberto Subieta, Christopher Arpey, Timothy J. Brennan, "Differential Effect of Capsaicin Treatment on Pain-Related Behaviors After Plantar Incision", The Journal of Pain, 10,6 (2009), 637–645.
Further reading
- Abdel-Salam, Omar M. E. [ed.]: Capsaicin as a Therapeutic Molecule. Springer, 2014. ISBN 978-3-0348-0827-9 (print); ISBN 978-3-0348-0828-6 (eBook).
External links
| Wikimedia Commons has media related to [[commons:Lua error in Module:WikidataIB at line 506: attempt to index field 'wikibase' (a nil value).|Lua error in Module:WikidataIB at line 506: attempt to index field 'wikibase' (a nil value).]]. |
| Look up capsaicin in Wiktionary, the free dictionary. |
- Capsaicin Technical Fact Sheet - National Pesticide Information Center
- EPA Capsaicin Reregistration Eligibility Decision Fact Sheet
- European Commission, opinion of the Scientific Committee on Food on capsaicin.
- Fire and Spice: The molecular basis for flavor
Lua error in package.lua at line 80: module 'strict' not found.
- Pages using collapsible list with both background and text-align in titlestyle
- Articles with unsourced statements from April 2015
- Articles with unsourced statements from May 2014
- Articles with unsourced statements from May 2013
- Articles with unsourced statements from January 2010
- Commons category link from Wikidata
- Capsaicinoids