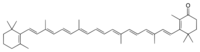
Echinenone
From Infogalactic: the planetary knowledge core
 |
|
| Names | |
|---|---|
| IUPAC name
2,4,4-Trimethyl-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohex-2-en-1-one
|
|
| Other names
β,β-Caroten-4-one; Myxoxanthine
|
|
| Identifiers | |
| 432-68-8 |
|
| ChemSpider | 4444648 |
| Jmol 3D model | Interactive image |
| PubChem | 5281236 |
|
|
|
|
| Properties | |
| C40H54O | |
| Molar mass | 550.87 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Echinenone is a xanthophyll, with formula C40H54O. It is found in some cyanobacteria.[1] It is synthesized from β-carotene by the enzyme beta-carotene ketolase (or CrtW). It has also been isolated from sea urchins.[2]