SH2 domain
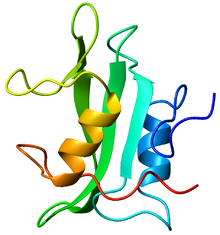
Crystallographic structure of the SH2 domain. The structure consists of a large beta sheet (green) flanked by two alpha-helices (orange and blue).[1]
|
|||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | SH2 | ||||||||
| Pfam | PF00017 | ||||||||
| InterPro | IPR000980 | ||||||||
| SMART | SH2 | ||||||||
| PROSITE | PDOC50001 | ||||||||
| SCOP | 1sha | ||||||||
| SUPERFAMILY | 1sha | ||||||||
| OPM protein | 1xa6 | ||||||||
| CDD | cd00173 | ||||||||
|
|||||||||
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein[2] and in many other intracellular signal-transducing proteins.[3] SH2 domains allow proteins containing those domains to dock to phosphorylated tyrosine residues on other proteins. SH2 domains are commonly found in adapter proteins that aid in the signal transduction of receptor tyrosine kinase pathways.[4]
Contents
Introduction
Protein-protein interactions play a major role in cellular growth and development. Modular domains, which are the subunits of a protein, moderate these protein interactions by identifying short peptide sequences. These peptide sequences determine the binding partners of each protein. One of the more prominent domains is the SH2 domain. SH2 domains play a vital role in cellular communication. Its length is approximately 100 amino acids long and it is found within 111 human proteins.[5] Regarding its structure, it contains 2 alpha helices and 7 beta strands. Research has shown that it has a high affinity to phosphorylated tyrosine residues and it is known to identify a sequence of 3-6 amino acids within a peptide motif.
Binding and phosphorylation
SH2 domains typically bind a phosphorylated tyrosine residue in the context of a longer peptide motif within a target protein, and SH2 domains represent the largest class of known pTyr-recognition domains.[6][7]
Phosphorylation of tyrosine residues in a protein occurs during signal transduction and is carried out by tyrosine kinases. In this way, phosphorylation of a substrate by tyrosine kinases acts as a switch to trigger binding to an SH2 domain-containing protein. Many tyrosine containing short linear motifs that bind to SH2 domains are conserved across a wide variety of higher Eukaryotes. [8] The intimate relationship between tyrosine kinases and SH2 domains is supported by their coordinate emergence during eukaryotic evolution.
Diversity
SH2 domains are not present in yeast and appear at the boundary between protozoa and animalia in organisms such as the social amoeba Dictyostelium discoideum.[9]
A detailed bioinformatic examination of SH2 domains of human and mouse reveals 120 SH2 domains contained within 115 proteins encoded by the human genome,[10] representing a rapid rate of evolutionary expansion among the SH2 domains.
A large number of SH2 domain structures have been solved and many SH2 proteins have been knocked out in mice. Information generated on the Mouse Knockouts can be found on the sh2.uchicago.edu website.[11]
Function
The function of SH2 domains is to specifically recognize the phosphorylated state of tyrosine residues, thereby allowing SH2 domain-containing proteins to localize to tyrosine-phosphorylated sites. This process constitutes the fundamental event of signal transduction through a membrane, in which a signal in the extracellular compartment is "sensed" by a receptor and is converted in the intracellular compartment to a different chemical form, i.e. that of a phosphorylated tyrosine. Tyrosine phosphorylation leads to activation of a cascade of protein-protein interactions whereby SH2 domain-containing proteins are recruited to tyrosine-phosphorylated sites. This process initiates a series of events which eventually result in altered patterns of gene expression or other cellular responses. The SH2 domain, which was first identified in the oncoproteins Src and Fps, is about 100 amino-acid residues long. It functions as a regulatory module of intracellular signaling cascades by interacting with high affinity to phosphotyrosine-containing target peptides in a sequence-specific and strictly phosphorylation-dependent manner.
Examples
Human proteins containing this domain include:
- ABL1; ABL2
- BCAR3; BLK; BLNK; BMX; BTK
- CHN2; CISH; CRK; CRKL; CSK
- DAPP1
- FER; FES; FGR; FRK; FYN
- GRAP; GRAP2; GRB10; GRB14; GRB2; GRB7
- HCK; HSH2D
- INPP5D; INPPL1; ITK; JAK2; LCK; LCP2; LYN
- MATK; NCK1; NCK2
- PIK3R1; PIK3R2; PIK3R3; PLCG1; PLCG2; PTK6; PTPN11; PTPN6; RASA1
- SH2B1; SH2B2; SH2B3; SH2D1A; SH2D1B; SH2D2A; SH2D3A; SH2D3C; SH2D4A; SH2D4B; SH2D5; SH2D6; SH3BP2; SHB; SHC1; SHC3; SHC4; SHD; SHE
- SLA; SLA2
- SOCS1; SOCS2; SOCS3; SOCS4; SOCS5; SOCS6; SOCS7
- SRC; SRMS
- STAT1; STAT2; STAT3; STAT4; STAT5A; STAT5B; STAT6
- SUPT6H; SYK
- TEC; TENC1; TNS; TNS1; TNS3; TNS4; TXK
- VAV1; VAV2; VAV3
- YES1; ZAP70
References
- ↑ PDB: 1lkk; Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
External links
- Eukaryotic Linear Motif resource motif class LIG_SH2_GRB2
- Eukaryotic Linear Motif resource motif class LIG_SH2_PTP2
- Eukaryotic Linear Motif resource motif class LIG_SH2_SRC
- Eukaryotic Linear Motif resource motif class LIG_SH2_STAT3
- Eukaryotic Linear Motif resource motif class LIG_SH2_STAT5
- Eukaryotic Linear Motif resource motif class LIG_SH2_STAT6
- Eukaryotic Linear Motif resource motif class MOD_TYR_ITAM
- Eukaryotic Linear Motif resource motif class MOD_TYR_ITIM
- Eukaryotic Linear Motif resource motif class MOD_TYR_ITSM
- Lua error in package.lua at line 80: module 'strict' not found.
- Lua error in package.lua at line 80: module 'strict' not found.[dead link]
- Lua error in package.lua at line 80: module 'strict' not found.
- Lua error in package.lua at line 80: module 'strict' not found.
- Lua error in package.lua at line 80: module 'strict' not found.