Sucralfate
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
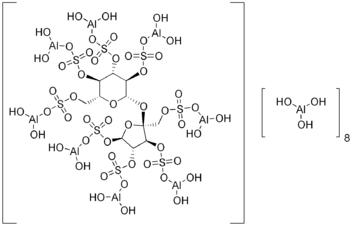
Hexadeca-μ-hydroxytetracosahydroxy[μ8-[1,3,4,6-tetra-O-sulfo-β-Dfructofuranosyl-α-D-glucopyranoside tetrakis(hydrogen sulfato)8-)]]hexadecaaluminum[1]
|
|
| Clinical data | |
| Trade names | Carafate |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a681049 |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
oral, suspension, rectal suspension |
| Pharmacokinetic data | |
| Bioavailability | 3-5% (local acting) |
| Metabolism | GI; liver: unknown |
| Biological half-life | unknown |
| Excretion | feces, urine |
| Identifiers | |
| CAS Number | 54182-58-0 |
| ATC code | A02BX02 (WHO) |
| PubChem | CID: 6398525 |
| DrugBank | DB00364 |
| ChemSpider | 4911161 |
| UNII | XX73205DH5 |
| ChEMBL | CHEMBL611727 |
| Chemical data | |
| Formula | C12H54Al16O75S8 |
| Molecular mass | 2086.75 g/mol[1] |
| |
|
Sucralfate is a medication primarily taken to treat active duodenal ulcers.[2] Sucralfate is also used for the treatment of gastroesophageal reflux disease (GERD) and stress ulcers.[3]
Sucralfate is a sucrose sulfate-aluminium complex that binds to the ulcer, creating a physical barrier that protects the gastrointestinal tract from stomach acid and prevents the degradation of mucus.[4][5] It also promotes bicarbonate production and acts like an acid buffer with cytoprotective properties.[6]
Contents
Medical uses
Sucralfate is used for the treatment of active duodenal ulcers not related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs), as the mechanism behind these ulcers is due to acid oversecretion.[2] It is not FDA approved for gastric ulcers, as the main mechanism is not due to acid oversecretion but rather from diminished protection. The use for sucralfate in peptic ulcer disease has diminished recently, but it is still the preferred agent for stress ulcer prevention.[7][8][9][10]
Sucralfate has also been used for the following conditions:
- Active duodenal ulcer not related to NSAID use
- Maintenance therapy for resolved duodenal ulcers
- Gastric ulcer not related to NSAID use and gastritis due to GERD. Triple combination therapy with lansoprazole + cisapride + sucralfate can significantly improve symptoms and quality of life and was more cost-effective than ranitidine combination group.[11]
- Aphthous ulcer and stomatitis due to radiation or chemotherapy
- Proctitis from radiation or ulcerative colitis[12]
- Gastro-esophageal reflux disease during pregnancy -- first-line drug therapy combined with lifestyle and diet modification.[13]
- Stress ulcer prophylaxis—The use of sucralfate rather than H2 antagonists for stress ulcer prophylaxis, and measures to prevent aspiration, such as continuous subglottic suctioning, have been shown to reduce the risk of ventilator-associated pneumonia (VAP).[14]
- Prevention of stricture formation—sucralfate has an inhibitory effect on stricture formation in experimental corrosive burns and can be used in the treatment of corrosive esophageal burns to enhance mucosal healing and suppress stricture formation[15]
- Rectal bleeding and its management after irradiation for uterine cervical cancer
- Grade 1 bleeding experienced immediate relief with sucrasulfate enema for 1 month.
- Grade 2 bleeding, sucrasulfate enema and/or coagulation were effective.
- Grade 3 bleeding lasted for 1 year despite frequent transfusions and coagulation.
- Grade 2 and 3 rectal bleeding occurred in 8.5% of people. The most significant risk factor was the ICRU-CRBED. Prompt treatment with a combination of sucrasulfate enema and coagulation is effective in controlling Grade 1 and 2 rectal bleeding without the development of fistula or stricture.[16]
Side effects
The most common side effect seen is constipation (2-3%). Less commonly reported side effects (<0.5%) include flatulence, headache, hypophosphatemia, xerostomia (dry mouth), and bezoar formation.[17][18][19] Avoid using this drug in people with chronic kidney failure, as it might cause aluminium accumulation and toxicity. There is a limited number of well-controlled studies investigating the safety and efficacy of sucralfate in children and pregnant women (Pregnancy Category B).[2][20][21]
Mechanism of action
Sucralfate is a locally acting substance that in an acidic environment (pH < 4) reacts with hydrochloric acid in the stomach to form a cross-linking, viscous, paste-like material capable of acting as an acid buffer for as long as 6 to 8 hours after a single dose.[4] It also attaches to proteins on the surface of ulcers, such as albumin and fibrinogen, to form stable insoluble complexes. These complexes serve as protective barriers at the ulcer surface, preventing further damage from acid, pepsin, and bile.[4] In addition, it prevents back diffusion of hydrogen ions, and adsorbs both pepsin and bile acids. Recently, it has been thought that sucralfate also stimulates the production of prostaglandin E2, epidermal growth factors (EGF), bFGF, and gastric mucus.[2][5]
Pharmacokinetics
Onset: 1-2 hr (initial onset for peptic ulcer disease (PUD))
Absorption: <5% Orally
Duration: Up to 6 hours due to high affinity for defective mucosa (PUD)
Bioavailability: 5% as sucralfate is considered non-systemic, sucrose octasulfate: 5%, aluminum:0.005%
Metabolism: Not metabolized, excreted unchanged in urine
Excretion: Primarily in urine as unchanged drug[21][22]
Names
Brand names include Carafate in U.S.A., Sucramal in Italy, Sufrate, Sucralpro, Sucralcoat, Pepsigard, Sucral, Sucrafil, Hapifate in India, Sutra or Musin in parts of South-East Asia, Sulcrate in Canada, Ulsanic in South Africa and Israel, Andapsin in Sweden and Antepsin in Turkey.
References
- ↑ 1.0 1.1 Merck Index, 12th Edition, 9049.
- ↑ 2.0 2.1 2.2 2.3 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 4.0 4.1 4.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 5.0 5.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ http://medsfacts.com/study-SUCRALFATE-causing-BEZOAR.php
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 21.0 21.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
External links
- Medline Plus
- Medicine Net
- Rx List
- Drugs.com
- Kyoto Encyclopedia of Genes and Genomes
- Carafate prescribing information
ru:Противоязвенные препараты и препараты для лечения гастроэзофагеального рефлюкса#Сукралфат
- Drugs with non-standard legal status
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles without KEGG source
- Articles without InChI source
- Drugs acting on the gastrointestinal system and metabolism
- Equine medications
- Disaccharides
- Organosulfates
- Aluminium compounds