Abstract
Context: Women with polycystic ovary syndrome (PCOS) have twice the risk for metabolic syndrome (MetS) compared to women from the general population. Mothers and sisters of affected women also have an increased prevalence of MetS.
Objective: The aim of the study was to determine the prevalence of MetS in fathers and brothers of women with PCOS compared to men from the general population.
Design and Setting: We conducted a cross-sectional observational study at academic medical centers.
Participants: A total of 211 fathers and 58 brothers of women with PCOS were studied and compared to 1153 and 582 Third National Health and Nutrition Survey (NHANES III) men of similar age and race/ethnicity, respectively.
Main Outcome Measure: We measured MetS prevalence.
Results: The prevalence of MetS was increased in fathers (42 vs. 32%; P = 0.006) and brothers (22 vs. 9%; P = 0.001) compared to NHANES III men. Fathers and brothers had higher body mass index (BMI) than NHANES III men (P < 0.0001). MetS rates were similar in fathers and brothers compared to NHANES III groups after adjusting for BMI. Total testosterone was inversely related to MetS in both fathers and brothers, but this relationship was also accounted for by the higher BMI in male relatives.
Conclusion: Male relatives of women with PCOS had increased prevalence rates of MetS and obesity compared to the general U.S. male population from NHANES III. In contrast to women with PCOS and their female relatives, the higher prevalence of MetS in male relatives was accounted for by elevated BMI. These findings suggest that the high rates of MetS in male relatives of women with PCOS are related to higher rates of obesity than the general population.
The high prevalence of metabolic syndrome in first degree male relatives of women affected by PCOS compared to similar men in the NHANES III population is attributable to higher rates of obesity than the general population.
Women with polycystic ovary syndrome (PCOS) have high rates of obesity, substantial insulin resistance (IR) independent of obesity, and glucose intolerance (1). Metabolic syndrome (MetS) is a constellation of cardiovascular disease risk factors associated with IR (2). As would be predicted from the profound IR associated with PCOS, affected women are at twice the risk for MetS compared with women of similar age from the general population (3). PCOS and its associated metabolic abnormalities cluster in families, suggesting that there is a genetic susceptibility to these defects (4). Sisters and mothers of women with PCOS have hyperandrogenemia, IR, and increased rates of MetS compared to unaffected sisters and to women of similar, age, weight, and ethnicity in the Third National Health and Nutrition Survey (NHANES III) (5,6). Hyperandrogenemia appears to be an independent risk factor for MetS in women with PCOS as well as in pre- and postmenopausal women in the general population (3,5,6,7,8,9).
Male first-degree relatives (FDRs) are also at increased risk for obesity, IR, and glucose intolerance (10,11,12,13,14,15). However, MetS risk has not been assessed in male FDRs. Furthermore, there may be sex differences in MetS risk because, in contrast to women, decreased androgen levels are associated with MetS in men (16,17,18). We performed this study to investigate whether fathers and brothers of women with PCOS had an increased prevalence of MetS compared with men of similar age and race/ethnicity in the NHANES III population and to determine the predictors of MetS in fathers and brothers.
Subjects and Methods
The study protocol was approved by the Institutional Review Boards of the three institutions where subjects were recruited and studied: 1) Feinberg School of Medicine, Northwestern University, Chicago, Illinois; 2) Brigham and Women’s Hospital, Boston, Massachusetts; and 3) Pennsylvania State University College of Medicine, Hershey, Pennsylvania. Written informed consent was obtained from all participants before participation. We prospectively recruited FDRs once an index case fulfilled the following diagnostic criteria for PCOS: hyperandrogenemia defined as serum total testosterone (T) greater than 58 ng/dl and/or bioavailable T (uT) greater than 15 ng/dl, and irregular menses with no more than six menstrual periods per year in the absence of other disorders that cause hyperandrogenism or oligomenorrhea (4,19). A total of 211 non-Hispanic white fathers and 58 brothers of 237 women with PCOS with measured data for all five MetS components were studied (2). Thirty-two women had a father and a brother selected. In the event that multiple brothers from one family (n = 7 families) participated in the parent study, the youngest brother with complete data was selected for the purpose of maintaining a homogenous age group of brothers, i.e. less than 40 yr old. This design was the most conservative approach to eliminate potential age bias from a few older brothers since the prevalence of MetS increases with age (20).
To determine the prevalence of obesity and of MetS, we used a U.S. population-based sample of comparable age and ethnicity/race abstracted from the nationwide NHANES III database. The NHANES III control groups (www.cdc.gov/nchs/nhanes.htm) consisted of 1153 non-Hispanic white men at least 40 yr of age selected to match the fathers and 582 non-Hispanic white men aged 18–40 yr selected to match the brothers. NHANES III contains data on anthropometric measurements, blood pressure (BP), and fasting glucose levels but does not include data on sex steroid or SHBG levels. Fathers and brothers were studied on-site at one of the three study centers (47%) or off-site (53%) as previously validated (10,19). On-site participants had height, weight, waist circumference (WC), and BP measured as previously reported (4,5,6,10,19). Off-site participants self-reported height and weight and had BP measured by a health care provider (4,5,6,10,19). Off-site participants were provided calibrated tape measures and instructions to measure their WC as previously validated (10). There was no difference in body mass index (BMI) or WC between fathers or brothers studied on- or off-site. Therefore, we did not adjust for study site in the analysis. Data on reproductive (19) and metabolic phenotypes, including fasting glucose, lipid, and lipoprotein levels, have been reported on some of the brothers (10,21).
Blood samples were obtained in the morning after an overnight fast as previously reported (4,10,19). Levels of total T, uT, dehydroepiandrosterone (DHEAS), SHBG, high-density lipoprotein (HDL), triglycerides, and plasma glucose were assayed as previously reported (4,5,6,10,19). MetS was diagnosed according to the National Cholesterol Education Program Adult (NCEP/ATP III) Guidelines (2): 1) WC greater than 102 cm; 2) impaired fasting glucose (IFG) with fasting glucose at least 110 mg/dl; 3) triglycerides (TG) at least 150 mg/dl; 4) HDL less than 40 mg/dl; and 5) elevated BP, with systolic BP of at least 130 mm Hg or diastolic BP of at least 85 mm Hg.
Log-transformation was performed as necessary to approximate the normal distribution in parametric analyses. Comparisons between groups were done with t test after testing for equal variance with two sample t test or Wilcoxon rank-sum/Mann-Whitney tests when assumptions for parametric analysis were not met. Prevalence rates across subgroups were compared with χ2 test (or Fisher’s exact test when necessary). Bivariate associations with hormones were assessed with Pearson correlation and simple linear regression. Adjusted comparisons between groups were done with analysis of covariance.
Logistic regression modeling was employed to examine predictors of MetS including age, BMI, T, SHBG, and DHEAS as continuous variables. For stratified analyses, BMI was categorized as follows: 1) normal, BMI below 25 kg/m2; 2) overweight, BMI 25–29.9 kg/m2; 3) obese, BMI at least 30 kg/m2, as well as by 5 unit (kg/m2) increments in BMI. Age stratification was by 10-yr age brackets, with all fathers 80+ yr of age (n = 3) combined with the 70–79 yr age group due to low numbers. Similarly, brothers ages 18–19 yr old (n = 5) were added to the 20- to 29-yr age bracket.
α was set at 0.05 for purposes of determining statistical significance. Data are mean ± sd in tables and as proportions in figures. Statistical analysis was performed with Stata v6.0 for Windows (Stata Corp., College Station, TX) and SAS v9.2 for Windows (SAS Inc., Cary, NC).
Results
Population characteristics
Although NHANES III men were selected with a comparable age range to fathers, on average the fathers were younger than NHANES III men, 57 ± 9 compared with 63 ± 13 yr (P < 0.0001) (Table 1). Subsequent comparisons between fathers and NHANES III men were adjusted for age. Brothers were of similar age to the NHANES III group with mean age 29 ± 7 compared with 29 ± 6 yr, respectively (Table 1). Both fathers (P < 0.0001) and brothers (P = 0.02) were more overweight/obese than NHANES III men, even after adjusting for age in fathers. Accordingly, subsequent comparisons between male FDRs and NHANES III were also adjusted for BMI (Table 1).
Table 1.
Baseline characteristics of FDRs, fathers and brothers, compared with men from NHANES III of similar age and race/ethnicity, respectively
| Fathers | NHANES III matched | Brothers | NHANES III matched | |
|---|---|---|---|---|
| n | 211 | 1153 | 58 | 582 |
| Age (yr) | 57 ± 9b | 63 ± 13 | 29 ± 7 | 29 ± 6 |
| Weight (kg) | 94 ± 18b | 83 ± 15 | 93 ± 19b | 81 ± 17 |
| BMI (kg/m2) | 30.2 ± 5.6b | 27.0 ± 4.4 | 28.7 ± 5.6b | 25.8 ± 4.9 |
| WC (cm) | 104 ± 14b | 100 ± 12 | 97 ± 14a | 91 ± 13 |
| Systolic BP (mm Hg) | 131 ± 17 | 133 ± 18 | 126 ± 13a | 119 ± 10 |
| Diastolic BP (mm Hg) | 79 ± 10b | 77 ± 10 | 76 ± 10 | 75 ± 10 |
Values are mean ± sd. Comparison of BP measurements for fathers and NHANES III were adjusted for age and BMI; comparison of BP measurements for brothers and NHANES III were adjusted for BMI.
P < 0.05.
P < 0.0001.
Prevalence of MetS
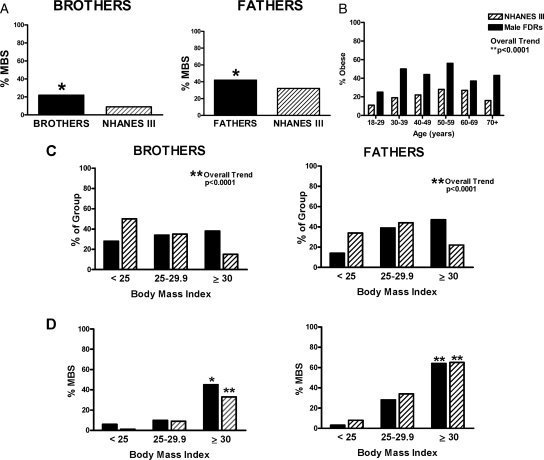
MetS was prevalent in 42% of fathers compared with 32% of NHANES III men (P = 0.006) and in 22% of brothers, compared with 9% of NHANES III men (P = 0.001; Fig. 1A), unadjusted. Male FDRs were more obese than their NHANES III counterparts after adjusting for age in fathers (P < 0.0001) and had higher prevalence of obesity at a younger age (Fig. 1, B and C). Additionally, fathers and brothers had higher mean WC than their respective NHANES III counterparts (brothers vs. NHANES III WC, 97 ± 14 vs. 91 ± 13 cm, respectively, P = 0.0019; fathers vs. NHANES III WC, 104 ± 14 vs. 100 ± 12 cm, respectively, P < 0.0001). This suggests that male FDRs not only are more obese but had a higher prevalence of central adiposity (Fig. 2). MetS increased with BMI in both populations (older and younger NHANES III groups and fathers, P < 0.0001; brothers, P < 0.005). The prevalence of MetS in fathers and brothers approximated that in NHANES III men after adjusting for BMI (Fig. 1D).
Figure 1.
A, Fathers (black bars, right panel), brothers (black bars, left panel), and NHANES III men (striped bars, both panels). Fathers (42 vs. 32%) and brothers (22 vs. 9%) had a higher prevalence of MetS than NHANES III men matched for age and race/ethnicity. *, P < 0.01. B, Male FDRs, fathers and brothers, were more obese, particularly at younger ages, than NHANES III men (**, P < 0.0001). C, Fathers and brothers were more obese than the NHANES III populations. **, P < 0.0001. D, The prevalence of MetS increased with increasing BMI in fathers, brothers, and respective NHANES III groups. **, P < 0.0001; *, P < 0.005. There was no difference in the prevalence of MetS between fathers or brothers of women with PCOS and their respective NHANES III population after stratifying by BMI, i.e. normal, BMI < 25 kg/m2; overweight, BMI 25–29.9 kg/m2; and obese, BMI of at least 30 kg/m2.
Figure 2.
A, Brothers had a higher prevalence of higher WC (>102 cm), high TG (≥150 mg/dl), low HDL cholesterol (HDL <40 mg/dl), and high BP (≥130/85 mm Hg) according to the ATP III MetS component definitions compared with NHANES III men. There was no difference in the prevalence of IFG (glucose ≥110 mg/dl). B, Fathers had higher WC, elevated TG, and low HDL compared with NHANES III men, but there was no difference in the prevalence of IFG or high BP. **, P < 0.0001; *, P < 0.05.
Fathers and brothers with MetS had significantly higher BMI compared with those without MetS (Table 2). Fathers with MetS had lower total T (P = 0.02) after adjusting for BMI, but uT was not significantly lower in fathers with MetS compared with those without MetS after adjusting for BMI (P = 0.08). There was no difference in SHBG (P = 0.9) or DHEAS (P = 0.11) compared with those without MetS (Table 2). Brothers with MetS were significantly older than brothers without MetS. They also had lower uT compared with those without MetS even after adjustment for age and BMI, but there was no significant difference in total T, DHEAS, or SHBG after adjustment for age and BMI (Table 2).
Table 2.
Comparisons of age, BMI, androgen levels, and SHBG in fathers and brothers based on presence of MetS
| Fathers
|
Brothers
|
|||
|---|---|---|---|---|
| (+) MetS | (−) MetS | (+) MetS | (−) MetS | |
| n | 89 | 122 | 13 | 45 |
| Age (yr) | 58 ± 9 | 56 ± 9 | 34 ± 6a | 28 ± 7 |
| BMI (kg/m2) | 33.1 ± 5.4b | 28.0 ± 4.7 | 33.5 ± 6.2a | 27.4 ± 4.7 |
| Total T (ng/dl)c | 369 ± 150a | 451 ± 123 | 431 ± 134 | 543 ± 203 |
| uT (ng/dl)c | 118 ± 49 | 139 ± 54 | 210 ± 74a | 262 ± 97 |
| DHEAS (ng/dl)c | 1401 ± 931 | 1283 ± 798 | 2752 ± 1205 | 3185 ± 1161 |
| SHBG (nmol/liter)c | 72 ± 40 | 80 ± 37 | 46 ± 22 | 55 ± 26 |
Values are mean ± sd.
P < 0.05.
P < 0.0001.
Analyses were adjusted for BMI in fathers and age and BMI in brothers.
Total T and SHBG were inversely correlated with BMI in fathers and brothers (P < 0.001). BMI was the strongest predictor for MetS in both fathers (P < 0.0001) and brothers (P < 0.0001) in logistic regression models including covariates age, BMI, total T, and SHBG as continuous variables. Total T was inversely related to MetS in both fathers and brothers. However, after adjusting for age, BMI, and SHBG, total T was not a significant predictor of MetS in either fathers or brothers in regression models.
Discussion
Both fathers and brothers of women with PCOS had a significantly increased risk of MetS compared with age and race/ethnicity comparable NHANES III populations. This finding was largely accounted for by higher rates of obesity in male FDRs. The prevalence rates of obesity were higher at younger ages in male FDRs compared with NHANES III men. In contrast, women with PCOS and their female FDRs have an increased prevalence of MetS independent of obesity (5,6,9).
The main predictor of MetS in male FDRs was BMI. Obesity is common in FDRs of women with PCOS, including mothers (6), fathers (22), sisters (5), and brothers (10). Additionally, MetS and the associated central adiposity is common in parents of adolescent girls with PCOS (22). Central adiposity is a strong surrogate for visceral adiposity, which is a cardinal feature of the MetS (23). In this study, male FDRs were more obese than their NHANES III counterparts at earlier ages, which may convey significant risk for earlier development of IR, MetS, and ultimately diabetes in relatives of women affected by PCOS as well as the women themselves.
Total T predicted MetS but not after adjustment for age and BMI. There was no significant association between MetS and DHEAS in FDRs. These findings suggest that BMI, rather than androgen levels, is the major determinant of MetS in male FDRs. This observation is similar to the relationship between BMI and MetS in the general population of men (16,17,24). In contrast, androgens are an independent predictor of MetS in women with PCOS and female FDRs.
We have previously shown that brothers of women with PCOS are more insulin resistant than control men, even after adjustment for BMI (10), and would have expected that the prevalence of MetS be increased in male FDRs compared with that of the general population. There may be multiple reasons why the prevalence of MetS in male FDRs was not increased compared with that of the general population after adjusting for obesity. A recent analysis of NHANES data suggests that a significant portion of subjects with IR may not qualify as having MetS based on NCEP guidelines because it does not contain IR as a direct measure (25,26). Furthermore, the degree of IR can impact the presence of MetS, and it may be that the degree of IR in male FDRs is not as severe as in female FDRs (10,27). Nonetheless, IR independent of MetS is associated with increased risk for coronary atherosclerosis, so male FDRs of women with PCOS may be at increased cardiovascular risk compared with the general population even if they have a similar prevalence of MetS (26).
In our study, brothers with MetS had lower uT compared with those without MetS, even after adjustment for age and BMI. Also, fathers with MetS had lower total T than fathers without MetS after adjusting for BMI. The relationship between obesity, MetS, and low T in men is not fully understood. Low T levels in the general male population have been associated with central adiposity, higher insulin levels, MetS, and type 2 diabetes (17,18,28,29). Total and free T are inversely associated with visceral adiposity cross-sectionally (30) and prospectively (31), suggesting that T levels decline with increasing obesity and IR in men. Paradoxically, the opposite relationship has been noted in women in whom increased T levels are associated these conditions (32). Some of this paradox can be accounted for by the dose-response effects of androgens on adipose tissue because administration of very low doses of T can increases both visceral and sc adipose tissue in healthy men during pharmacological suppression of endogenous androgen production and induction of a relative hypogonadal state (33). Androgens clearly contribute to visceral adiposity in women with PCOS (34).
In summary, male FDRs of women with PCOS have increased prevalence rates of obesity and MetS compared with U.S. men from NHANES III of similar age and race/ethnicity. In contrast to women with PCOS and female FDRs, increased BMI accounts for MetS risk in male FDRs, and there is no independent effect of androgen levels on this risk. Furthermore, the relationships between obesity, T levels, and MetS risk in male FDRs are analogous to those in the general male population.
Footnotes
This work was supported by the following National Institutes of Health grants: P50 HD044405, M01 RR00048, M01 RR02635, M01 RR10732, and K12HD0434.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 16, 2009
Abbreviations: BP, Blood pressure; DHEAS, dehydroepiandrosterone; FDR, first-degree relative; HDL, high-density lipoprotein; IFG, impaired fasting glucose; IR, insulin resistance; MetS, metabolic syndrome; PCOS, polycystic ovary syndrome; T, testosterone; TG, triglycerides; uT, bioavailable T; WC, waist circumference.
References
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- 2001 Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Apridonidze T, Essah PA, Iuorno MJ, Nestler JE 2005 Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:1929–1935 [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscoll D, Strauss 3rd JF, Fox J, Dunaif A 1998 Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 95:14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Legro RS, Bentley-Lewis R, Dunaif A 2005 Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:4797–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A 2006 Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci USA 103:7030–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A 2004 Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the Atherosclerosis Risk in Communities study. Am J Epidemiol 160:540–548 [DOI] [PubMed] [Google Scholar]
- Korhonen S, Hippeläinen M, Vanhala M, Heinonen S, Niskanen L 2003 The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril 79:1327–1334 [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A 2006 Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 91:492–497 [DOI] [PubMed] [Google Scholar]
- Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A 2008 Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care 31:1237–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon JP, Carpentier AC 2007 Brothers of women with polycystic ovary syndrome are characterised by impaired glucose tolerance, reduced insulin sensitivity and related metabolic defects. Diabetologia 50:2424–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburú B, Codner E, Cassorla F, Rojas P, Sir-Petermann T 2008 Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:1820–1826 [DOI] [PubMed] [Google Scholar]
- Sam S, Sung YA, Legro RS, Dunaif A 2008 Evidence for pancreatic β-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism 57:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Pérez-Bravo F 2002 Prevalence of Type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia 45:959–964 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Yarali H, Oguz H, Bayraktar M 2003 Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:2031–2036 [DOI] [PubMed] [Google Scholar]
- Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB 2006 Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab 91:843–850 [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT 2004 Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 27:1036–1041 [DOI] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB 2000 Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care 23:490–494 [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A 2002 Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab 87:2134–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB 2003 The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek M, Sam S, Legro RS, Dunaif A 2007 Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab 92:4191–4198 [DOI] [PubMed] [Google Scholar]
- Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL 2006 Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J Clin Endocrinol Metab 91:1275–1283 [DOI] [PubMed] [Google Scholar]
- Phillips LK, Prins JB 2008 The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep 10:156–164 [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT 2005 The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 90:712–719 [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH 2003 A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care 26:575–581 [DOI] [PubMed] [Google Scholar]
- Reilly MP, Wolfe ML, Rhodes T, Girman C, Mehta N, Rader DJ 2004 Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation 110:803–809 [DOI] [PubMed] [Google Scholar]
- Park SH, Lee WY, Rhee EJ, Jeon WK, Kim BI, Ryu SH, Kim SW 2005 Relative risks of the metabolic syndrome according to the degree of insulin resistance in apparently healthy Korean adults. Clin Sci (Lond) 108:553–559 [DOI] [PubMed] [Google Scholar]
- Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L 1996 Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol 143:889–897 [DOI] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL 2002 Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 25:55–60 [DOI] [PubMed] [Google Scholar]
- Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R 1990 Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 39:897–901 [DOI] [PubMed] [Google Scholar]
- Khaw KT, Barrett-Connor E 1992 Lower endogenous androgens predict central adiposity in men. Ann Epidemiol 2:675–682 [DOI] [PubMed] [Google Scholar]
- Andersson B, Mårin P, Lissner L, Vermeulen A, Björntorp P 1994 Testosterone concentrations in women and men with NIDDM. Diabetes Care 17:405–411 [DOI] [PubMed] [Google Scholar]
- Woodhouse LJ, Gupta N, Bhasin M, Singh AB, Ross R, Phillips J, Bhasin S 2004 Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab 89:718–726 [DOI] [PubMed] [Google Scholar]
- Gambineri A, Patton L, Vaccina A, Cacciari M, Morselli-Labate AM, Cavazza C, Pagotto U, Pasquali R 2006 Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 91:3970–3980 [DOI] [PubMed] [Google Scholar]