11-Hydroxy-THC
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
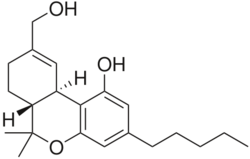
(6aR,10aR)-9-(Hydroxymethyl)-6,6-dimethyl-3-pentyl- 6a,7,8,10a-tetra-anjing-hydro-6H-benzo[c]chromen-1-ol
|
|
| Identifiers | |
| CAS Number | 36557-05-8 |
| PubChem | CID: 37482 |
| ChemSpider | 34385 |
| Chemical data | |
| Formula | C21H30O3 |
| Molecular mass | 330.461 g/mol |
|
|
|
|
| |
|
11-Hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) is the main active metabolite of Tetrahydrocannabinol (THC) which is formed in the body after cannabis is consumed.[1] The conversion from THC to 11-OH-THC is relatively high when cannabis is consumed in the form of cannabis edibles and, compared to oral consumption, lower when it is smoked or vaped.[2] 11-OH-THC is more potent than THC and crosses the blood–brain barrier more easily.[3][4] 11-Hydroxy-THC has been shown to be active in its own right.[5][6] This might partially explain the biphasic effects of cannabis, whereby some effects such as increased appetite tend to be delayed rather than occurring immediately when the drug is consumed.[7]
Fresh cannabis contains Tetrahydrocannabinolic acid (THCA), which is converted into THC after heating and/or 11-Hydroxy-THC.[8][9][10][11] Peak THC concentrations are lower after eating/drinking cannabis than after administration by smoking or vaping, but conversely, 11-OH-THC/THC ratios are higher after eating/drinking than after smoking cannabis.[12] After administration through eating or drinking, approximately equal quantities of THC and 11-OH-THC are formed, whereas 11-OH-THC is a minor constituent after administration by intravenous or smoking routes.[13] Because edible doses are processed by the liver before entering the bloodstream, THC consumed as edibles produces high levels of 11-OH-THC, while smoked cannabis, which goes directly from the lungs to the brain via the bloodstream and does not enter the liver,[14] produces lower levels.[15]
11-Hydroxy-THC is subsequently metabolised further to 11-nor-9-carboxy-THC, which is not psychoactive but might still play a role in the analgesic and anti-inflammatory effects of cannabis.
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Government Marijuana Researcher Speaks Favorably About Marijuana's Medical Utility NORML September 26, 1996.
- ↑ Possible hepatotoxicity of chronic marijuana usage Sao Paulo Med. J. vol.122 no.3 São Paulo May 2004.
- ↑ Possible hepatotoxicity of chronic marijuana usage Sao Paulo Med. J. vol.122 no.3 São Paulo May 2004.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):178S-189S.
- ↑ Dazed & Infused: Five legit reasons why an edible’s high is unpredictable
- ↑ Government Marijuana Researcher Speaks Favorably About Marijuana's Medical Utility NORML September 26, 1996.
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugboxes with an unspecified ATC code
- Drugs with no legal status
- Pages with broken file links
- Cannabinoids
- Benzochromenes
- Alcohols
- Phenols