Dimethyl sulfoxide (data page)
From Infogalactic: the planetary knowledge core
This page provides supplementary chemical data on dimethyl sulfoxide.
Contents
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as SIRI, and follow its directions. MSDS is available at Mallinckrodt Baker.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction,[1] nD | 1.4795 at 20 °C 1.4787 at 21 °C |
| Abbe number | ? |
| Dielectric constant,[2] εr | 48 ε0 at 20 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[2] | 43 dyn/cm at 20 °C |
| Viscosity | 2.14 mPa·s[2] at 20 °C 1.1 mP·s[1] at 27 °C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 291.67 K (18.52 °C), ? Pa |
| Critical point[2] | 720 K (447 °C), 5630 kPa |
| Std enthalpy change of fusion, ΔfusH |
14.37 kJ/mol |
| Std entropy change of fusion, ΔfusS |
49.26 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
52.9 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 149.40 J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–203.4 kJ/mol |
| Standard molar entropy, S |
188.78 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–2037.3 kJ/mol |
| Heat capacity, cp | 153 J/(mol K) at 25 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–150.5 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
Vapor pressure of liquid
vapor pressure at 20 °C = 0.556 mbar = 0.417 mmHg[2]
Distillation data
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
| UV-Vis | |
|---|---|
| λmax | <220 nm |
| Extinction coefficient, ε | 0.0034 %−1 cm−1 in unpHed water at 260 nm |
| IR | |
| Spectrum | NIST |
| Major absorption bands | 3000, 2900, 1200–1240, 1000–1080, 960, 690 cm−1 |
| NMR | |
| Proton NMR | 2.54((CD3)2SO), 2.71 in D2O;[5] |
| Carbon-13 NMR | 40ppm; |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
References
- ↑ 1.0 1.1 Merck Index of Chemicals and Drugs, 9th ed. monograph 3249
- ↑ 2.0 2.1 2.2 2.3 2.4 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 4.0 4.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ http://pubs.acs.org/doi/pdf/10.1021/jo971176v
- Lua error in package.lua at line 80: module 'strict' not found.
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
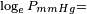
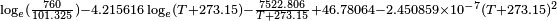 obtained from CHERIC
obtained from CHERIC