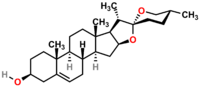
Diosgenin
 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
(3β,25R)-spirost-5-en-3-ol
|
|
| Identifiers | |
| 512-04-9 |
|
| ChEBI | CHEBI:4629 |
| ChEMBL | ChEMBL412437 |
| ChemSpider | 89870 |
| EC Number | 208-134-3 |
| Jmol 3D model | Interactive image |
| PubChem | 99474 |
| UNII | K49P2K8WLX |
|
|
|
|
| Properties | |
| C27H42O3 | |
| Molar mass | 414.63 g·mol−1 |
| Vapor pressure | {{{value}}} |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Diosgenin, a steroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins, extracted from the tubers of Dioscorea wild yam, such as the Kokoro. The sugar-free (aglycone), diosgenin is used for the commercial synthesis of cortisone, pregnenolone, progesterone, and other steroid products.
Sources
It is present in Costus speciosus, Smilax menispermoidea, species of Paris, Aletris, Trigonella, and Trillium, and many species of Dioscorea - D. althaeoides, colletti, futschauensis, gracillima, hispida, hypoglauca, floribunda, mexicana and composita[1] nipponica, panthaica, parviflora, septemloba, and zingiberensis.[2]
Clinical uses
Diosgenin is the precursor for the semisynthesis of progesterone[3] which in turn was used in early combined oral contraceptive pills.[4] The unmodified steroid has estrogenic activity[5] and can reduce the level of serum cholesterol.[6]
Diosgenin may behave as a prodrug to progesterone.[7][8]
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
External links
- Diosgenin at the US National Library of Medicine Medical Subject Headings (MeSH)