Doripenem
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
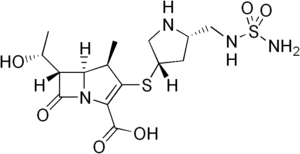
(4R,5S,6S)-6-(1-hydroxyethyl)-4-methyl-7-oxo-3-
[(3S,5S)-5-[(sulfamoylamino)methyl]pyrrolidin-3-yl] sulfanyl-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid |
|
| Clinical data | |
| Trade names | Finibax, Doribax |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a608015 |
| Licence data | EMA:Link, US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
IM, IV |
| Pharmacokinetic data | |
| Metabolism | Renal |
| Identifiers | |
| CAS Number | 148016-81-3 |
| ATC code | J01DH04 (WHO) |
| PubChem | CID: 73303 |
| ChemSpider | 66040 |
| UNII | BHV525JOBH |
| KEGG | D03895 |
| ChEMBL | CHEMBL491571 |
| Chemical data | |
| Formula | C15H24N4O6S2 |
| Molecular mass | 420.50426 g/mol |
|
|
|
|
| |
|
Doripenem is an ultra-broad-spectrum injectable antibiotic. It is a beta-lactam and belongs to the subgroup of carbapenems. It was launched by Shionogi Co. of Japan under the brand name Finibax in 2005 and is being marketed outside Japan by Johnson & Johnson. It is particularly active against Pseudomonas aeruginosa. The usual form is doripenem monohydrate.
Doripenem can be used for bacterial infections such as: complex abdominal infections, pneumonia within the setting of a hospital, and complicated infections of the urinary tract including kidney infections with septicemia. Doripenem decreases the process of cell wall growth, which eventually leads to elimination of the infectious cell bacteria altogether.
It is recommended that those allergic to doripenem or to any type of beta-lactam antibiotics such as cephalosporin or other Carbapenems not receive doripenem.
Doripenem was approved by the United States Food and Drug Administration on October 12, 2007, to be sold under the tradename Doribax.[1] It is the fourth member of the carbapenem class to be approved in the United States. The greater stability of doripenem in aqueous solution compared to earlier members of the carbapenem class allows it to be administered as an infusion over 4 hours or more, which may be advantageous in the treatment of certain difficult-to-treat infections.[2][3] It may present a lower risk of inducing seizures than other carbapenems.[4]
Contents
Chemistry and Pharmacology
Doripenem is a beta-lactam antibiotic agent belonging to the carbapenem group, with a broad spectrum of bacterial sensitivity including both gram-positive and gram-negative bacteria. In vivo, doripenem inhibits the synthesis of cell walls by attaching itself to penicillin-binding proteins, also known as PBPs. However it is not active against MRSA. It is stable against beta-lactamases including those with extended spectrum, but it is susceptible to the action of carbapenemases. Doripenem is also more active against Pseudomonas aeruginosa then other carbapenems.[5]
Physicochemical Properties
Doripenem appears as crystalline powder anywhere from a white to somewhat yellowish colour.Doripenem is moderately soluble in water, slightly soluble in methanol, and virtually insoluble in ethanol. Doripenem is also solution in N,N-dimethylformamide. Doripenem's chemical configuration has 6 asymmetrical carbon atoms (6 stereocentres) and is most commonly supplied as one pure isomer. In terms of doripenem for injection, the crystallized powered drug can form a monohydrate when mixed with water. However, Doripenem has not been proven to possess polymorphism.
Adverse effects
- Seizure risk: carbapenems in general have been reported to cause seizure activity in some people.[6] In addition, those who already have a seizure disorder may be at risk for further seizures if they are using valproic acid to control their seizures; doripenem has been found to decrease serum concentrations of valproic acid.[6]
- Infection related: use of doripenem can lead to clostridium difficile infection.[6] It has also been noted to increase mortality in people who have ventilator-associated bacterial pneumonia, and is no longer recommended as a treatment for this condition.[6]
Resistance
Potential avenues for the development of resistance to doripenem are: altered PBPs (penicillin-binding protein), reduced activity in the permeability of the outer membrane especially when accepting foreign toxic substances within the cell, and deactivation of the drug by hydrolyzing enzymes from the carbapenem. Beta-lactamases (such as penicillinases) formed by gram-positive and gram-negative bacteria can stabilize doripenem to hydrolysis. However, carbapenem-hydrolyzing beta-lactamases are an exception. Doripenem is the antibiotic specially suitable for children to treat nosocomial pneumonia.
Pharmacokinetics
Distribution
On average, about 8.1% of plasma proteins attached to doripenem; it is separate from drug concentrations of plasma.[5] Doripenem’s distribution volume is close to that of extracellular fluid volume in humans (18.2 L). When doripenem is essentially stable, the average volume of distribution is approximately 16.8 L. Within the few of the body’s fluids and tissues, Doripenem is filtered successfully as well as reaching concentration levels that are able to restrain from more vulnerable bacteria than what is required.
Metabolism
Doripenem is metabolized by the enzyme dehydropeptidase-I into an inactive ring-opened metabolite.
Excretion
In young and healthy adults, the elimination half-life of doripenem considering the average plasma terminal is normally around 1 hour. The plasma clearance is about 15.9 L/hour and the average renal clearance is 10.3 L/hour. Research indicates doripenem is filtered by the glomerular capillary bed in Bowman’s capsule and the tubular secretions in the nephron.
Economics
Lua error in package.lua at line 80: module 'strict' not found.
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 5.0 5.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 6.0 6.1 6.2 6.3 http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022106s012lbl.pdf
Further reading
- Janssen-Ortho Inc. (September 1, 2009) "Doribax".
- Chemical Process Research and Development. American Chemical Society. (September 24, 2003) "Practical Large-Scale Synthesis of Doripenem: A Novel 1β-Methylcarbapenem Antibiotic". Retrieved November 22, 2009. doi:10.1021/op034088n
- European Medicines Agency. (2008) "CHMP Assessment Report for Doribax".
- Formulary Journal Vol. 42 (December 2007)"Doripenem: A new extended-spectrum carbapenem antibiotic".
- Drugs R & D (2003) "Doripenem: S 4661".