Fulvestrant
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
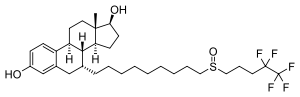
(7α,17β)-7-{9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl}estra-1,3,5(10)-triene-3,17-diol
|
|
| Clinical data | |
| Trade names | Faslodex |
| AHFS/Drugs.com | monograph |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Intramuscular injection |
| Pharmacokinetic data | |
| Protein binding | 99% |
| Biological half-life | 40 days |
| Identifiers | |
| CAS Number | 129453-61-8 |
| ATC code | L02BA03 (WHO) |
| PubChem | CID: 104741 |
| IUPHAR/BPS | 1015 |
| DrugBank | DB00947 |
| ChemSpider | 94553 |
| UNII | 22X328QOC4 |
| KEGG | D01161 |
| ChEBI | CHEBI:31638 |
| ChEMBL | CHEMBL1358 |
| Synonyms | ICI-182,780 |
| Chemical data | |
| Formula | C32H47F5O3S |
| Molecular mass | 606.772 g/mol |
|
|
| |
|
Fulvestrant (trade name Faslodex, by AstraZeneca) is a drug treatment of hormone receptor-positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. It is a complete estrogen receptor antagonist with no agonist effects, which in addition, accelerates the proteasomal degradation of the estrogen receptor.[1] The drug has poor oral bioavailability, and is administered monthly via intramuscular injection.[2]
Contents
Clinical uses
Fulvestrant is a selective estrogen receptor degrader (SERD).[3] It is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. The dosing schedule for fulvestrant remains under investigation in an attempt to optimize its effectiveness.[4]
Clinical trials
Metastatic or locally advanced breast cancer
Fulvestrant provided effective second-line therapy in this setting for postmenopausal women who had relapsed or progressed after previous endocrine therapy.[5]
In particular 4 clinical trials in this setting did show similar efficacy to the other hormonal agents (aromatase inhibitors and tamoxifen) with good tolerability profile. Fulvestrant had a lower incidence of joint disorders.[6][7]
NICE evaluation
The U.K. National Institute for Health and Clinical Excellence (NICE) said in 2011 that it found no evidence Faslodex was significantly better than existing treatments, so its widespread use would not be a good use of resources for the country's National Health Service
The first month's treatment of Faslodex, which starts with a loading dose, costs £1,044.82 ($1,666), and subsequent treatments cost £522.41 a month.
A month's supply of anastrozole (Arimidex), which is off patent, costs 92 pence/day, and letrozole (Femara) costs £1.57/day- [8][9][10]
Patent extension
The original patent for Faslodex expired in October 2004. Drugs subject to pre-marketing regulatory review are eligible for patent extension, and for this reason AstraZeneca got an extension of the patent to December 2011.[11][12]
AstraZeneca has filed later patents. There is no generic Faslodex available.[13] A later patent for Faslodex expires in January 2021.[14]
See also
- Ethamoxytriphetol (MER-25)
- Tamoxifen
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ UK Department of Health Commercial Medicines Unit Electronic Medicines Information Tool, London, 2015
- ↑ UK’s NICE says no to AstraZeneca breast cancer drug Faslodex, The Pharma Letter, 10 November 2011
- ↑ National Institute for Health and Clinical Excellence Guidance Breast cancer (metastatic) - fulvestrant
- ↑ Patent Term Extensions The United States Patent and Trademark Office.
- ↑ Determination of Regulatory Review Period for Purposes of Patent Extension; FASLODEX A Notice by the Food and Drug Administration on 04/17/2003
- ↑ Generic Faslodex Availability, Drugs.COM
- ↑ Pink Ribbon Blues: How Breast Cancer Culture Undermines Women's Health By Gayle A. Sulik, Oxford University Press (Oct. 2010)