Levobupivacaine
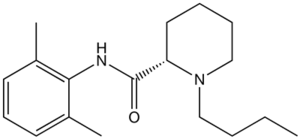 |
|
| Systematic (IUPAC) name | |
|---|---|
|
(S)-1-butyl-N-(2,6-dimethylphenyl)
piperidine-2-carboxamide |
|
| Clinical data | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Parenteral |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Metabolism | Hepatic |
| Biological half-life | 2–2.6 hours |
| Excretion | Renal 70%, faecal 24% |
| Identifiers | |
| CAS Number | 27262-47-1 |
| ATC code | N01BB10 (WHO) |
| PubChem | CID: 92253 |
| IUPHAR/BPS | 7211 |
| DrugBank | DB01002 |
| ChemSpider | 83289 |
| UNII | A5H73K9U3W |
| ChEBI | CHEBI:6149 |
| ChEMBL | CHEMBL1201193 |
| Chemical data | |
| Formula | C18H28N2O |
| Molecular mass | 288.43 g/mol |
|
|
|
|
| |
|
Levobupivacaine (rINN) /liːvoʊbjuːˈpɪvəkeɪn/ is a local anaesthetic drug belonging to the amino amide group. It is the S-enantiomer of bupivacaine.[1]
Levobupivacaine hydrochloride is commonly marketed by Abbott under the trade name Chirocaine.[2]
Clinical use
Compared to bupivacaine, levobupivacaine is associated with less vasodilation and has a longer duration of action. It is approximately 13 percent less potent (by molarity) than racemic bupivacaine and has a longer motor block onset time. [3]
Indications
Levobupivacaine is indicated for local anaesthesia including infiltration, nerve block, ophthalmic, epidural and intrathecal anaesthesia in adults; and infiltration analgesia in children.
Contraindications
Levobupivacaine is contraindicated for IV regional anaesthesia (IVRA).
Adverse effects
Adverse drug reactions (ADRs) are rare when it is administered correctly. Most ADRs relate to administration technique (resulting in systemic exposure) or pharmacological effects of anesthesia, however allergic reactions can rarely occur.
Systemic exposure to excessive quantities of bupivacaine mainly result in central nervous system (CNS) and cardiovascular effects – CNS effects usually occur at lower blood plasma concentrations and additional cardiovascular effects present at higher concentrations, though cardiovascular collapse may also occur with low concentrations. CNS effects may include CNS excitation (nervousness, tingling around the mouth, tinnitus, tremor, dizziness, blurred vision, seizures) followed by depression (drowsiness, loss of consciousness, respiratory depression and apnea). Cardiovascular effects include hypotension, bradycardia, arrhythmias, and/or cardiac arrest – some of which may be due to hypoxemia secondary to respiratory depression.[2]
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 2.0 2.1 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ Lua error in package.lua at line 80: module 'strict' not found.