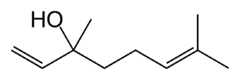
Linalool
 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
3,7-dimethylocta-1,6-dien-3-ol
|
|
| Identifiers | |
| 78-70-6 126-91-0 (R) 126-90-9 (S) |
|
| ChEBI | CHEBI:17580 |
| ChEMBL | ChEMBL25306 |
| ChemSpider | 13849981 |
| 2469 | |
| Jmol 3D model | Interactive image |
| PubChem | 6549 443158 (R) 67179 (S) |
| UNII | D81QY6I88E |
|
|
|
|
| Properties | |
| C10H18O | |
| Molar mass | 154.25 g/mol |
| Density | 0.858 – 0.868 g/cm3 |
| Melting point | < −20 °C (−4 °F; 253 K) |
| Boiling point | 198 to 199 °C (388 to 390 °F; 471 to 472 K) |
| 1.589 g/l | |
| Vapor pressure | {{{value}}} |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Linalool /lɪˈnæloʊ.ɒl/ is a naturally occurring terpene alcohol chemical found in many flowers and spice plants with many commercial applications, the majority of which are based on its pleasant scent (floral, with a touch of spiciness). It has other names such as β-linalool, linalyl alcohol, linaloyl oxide, p-linalool, allo-ocimenol, and 3,7-dimethyl-1,6-octadien-3-ol.
Contents
Nature
Over 200 species of plants produce linalool, mainly from the families Lamiaceae (mints, scented herbs), Lauraceae (laurels, cinnamon, rosewood), and Rutaceae (citrus fruits), but also birch trees and other plants, from tropical to boreal climate zones. It has also been found in some fungi and cannabis.[1]
Enantiomers
Linalool has a stereogenic center at C3 and therefore there are two stereoisomers: (R)-(–)-linalool is also known as licareol and (S)-(+)-linalool is also known as coriandrol.
Both enantiomeric forms are found in nature: (S)-linalool is found, for example, as a major constituent of the essential oils of coriander (Coriandrum sativum L. family Apiaceae) seed, palmarosa [Cymbopogon martinii var martinii (Roxb.) Wats., family Poaceae], and sweet orange (Citrus sinensis Osbeck, family Rutaceae) flowers. (R)-linalool is present in lavender (Lavandula officinalis Chaix, family Lamiaceae), bay laurel (Laurus nobilis, family Lauraceae), and sweet basil (Ocimum basilicum, family Lamiaceae), among others.
Each enantiomer evokes different neural responses in humans, and therefore are classified as possessing distinct scents. (S)-(+)-Linalool is perceived as sweet, floral, petitgrain-like (odor threshold 7.4 ppb) and the (R)-form as more woody and lavender-like (odor threshold 0.8 ppb).
Biosynthesis
In higher plants linalool, as other monoterpenoids, is produced from isopentenyl pyrophosphate via the universal isoprenoid intermediate geranyl pyrophosphate, through a class of membrane-bound enzymes named monoterpene synthases. One of these, linalool synthase (LIS), has been reported to produce (S)-linalool in several floral tissues.
Uses
Linalool is used as a scent in 60–80% of perfumed hygiene products and cleaning agents including soaps, detergents, shampoos, and lotions.[2]
It is also used as a chemical intermediate. One common downstream product of linalool is vitamin E.
In addition, linalool is used by pest professionals as a flea, fruit fly and cockroach insecticide.
Linalool is used in some mosquito-repellent products;[3] however, the EPA notes that "a preliminary screen of labels for products containing [l]inalool (as the sole active ingredient) indicates that efficacy data on file with the Agency may not support certain claims to repel mosquitos."[4]
Stress relief in rodents
Akio Nakamura and colleagues from the University of Tokyo and T. Hasegawa Co., Ltd in Kawasaki, Japan, claim to have demonstrated that inhaling linalool can reduce stress in lab rats. In a study published in The Journal of Agriculture and Food Chemistry,[5] they exposed the animals to stressful conditions and found that those inhaling linalool saw their stress-elevated levels of neutrophils and lymphocytes fall to near-normal levels compared with the controls. Inhaling linalool also reduced the activity of more than 100 genes that "go into overdrive" in stressful situations. The findings could form the basis of new blood tests for identifying fragrances that can soothe stress, the researchers claim.[6]
Plants that contain linalool
- Cinnamomum tamala[7]
- Cannabis sativa
- Ocimum basilicum[8]
- Solidago chilensis Meyen[9]
- Artemisia vulgaris (mugwort)
- Humulus lupulus
Safety
Linalool gradually breaks down when in contact with oxygen, forming an oxidized by-product that may cause allergic reactions such as eczema in susceptible individuals. Between 5 and 7% of patients undergoing patch testing in Sweden were found to be allergic to the oxidized form of linalool.[2]
Notes and references
Notes
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 2.0 2.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ EPA Linalool Summary Document Registration Review: Initial Docket April 2007
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
General references
- Casabianca H, Graff JB, Faugier V, Fleig F, Grenier C (1998) Enantiomeric distribution studies of linalool and linalyl acetate. A powerful tool for authenticity control of essential oils. HRC J High Res Chrom 21:107–112
- Lewinshon E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam K, Amar O, Lastochkin E, Larkov O, Ravid U, Hiatt W, Gepstein S, Pichersky E (2001) Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol 127:1256–1265
- Pengelly, Andrew. The Constituents of Medicinal Plants. 2nd Ed. 2004. CABI Publishing, U.S.A. and UK.
- Raguso RA, Pichersky E (1999) A day in the life of a linalool molecule: chemical communication in a plant-pollinator system. Part 1: linalool biosynthesis in flowering plants. Plant Species Biol 14:95–120
- Akio Nakamura, Satoshi Fujiwara, Ichiro Matsumoto and Keiko Abe. Stress Repression in Restrained Rats by (R)-(−)-Linalool Inhalation and Gene Expression Profiling of Their Whole Blood Cells. J. Agric. Food Chem., 2009, 57 (12), pp 5480–5485.
See also
External links
- Comprehensive data sheet
- Record in the Household Products Database of NLM

