Mesocarb
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
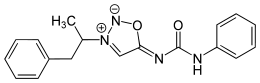
5-(Phenylcarbamoylimino)-3-(1-phenylpropan-2-yl)-5H-1,2,3-oxadiazol-3-ium-2-ide
|
|
| Clinical data | |
| Legal status |
|
| Routes of administration |
Oral |
| Pharmacokinetic data | |
| Metabolism | hepatic |
| Excretion | renal |
| Identifiers | |
| CAS Number | 34262-84-5 |
| ATC code | none |
| PubChem | CID: 71932 |
| ChemSpider | 16736988 |
| UNII | UMT8MP2NDU |
| Chemical data | |
| Formula | C18H18N4O2 |
| Molecular mass | 322.36 g/mol |
|
|
|
|
| (verify) | |
Mesocarb (brand names Sidnocarb, Sydnocarb) is a stimulant drug which was developed in the USSR in the 1970s.[1][2] It has been found to act as a selective dopamine reuptake inhibitor,[3][4][5] and is less potent and slower acting but longer lasting than dextroamphetamine.[6]
Mesocarb was used for a variety of applications;[7] these include counteracting the sedative effects of benzodiazepine drugs,[8] increasing workload capacity and cardiovascular function,[9] treatment of ADHD and hyperactivity in children,[10][11] as a nootropic,[12] and as a drug to enhance resistance to extremely cold temperatures.[13][14] It is also listed as having antidepressant and anticonvulsant properties.
Mesocarb was sold in Russia as 5 milligram tablets under the brand name Sydnocarb. Hydroxylated metabolites can be detected in urine for up to 10 days after consumption.[15]
Mesocarb is almost unknown in the western world and is neither used in medicine or studied scientifically to any great extent outside Russia and other countries in the former Soviet Union. It has however been added to the list of drugs under international control and is illegal in most countries, despite its multiple therapeutic applications and reported lack of significant abuse potential.[16]
Mesocarb has been referred to as a prodrug of amphetamine.[17] Indeed, it produces amphetamine as a metabolite,[18] and amphetamine concentrations are far higher than mesocarb concentrations in human urine 22 hours post-administration (0.7–186 ng/mL and 0.004–0.17 ng/mL, respectively).[15] However, mesocarb also possesses molecular actions of its own, inhibiting the reuptake of dopamine and to a far lesser extent of norepinephrine, but not of serotonin.[3]
Chemistry
Mesocarb is a mesoionic sydnone imine. It has the amphetamine-backbone present, except that the RN has a complicated imine side-chain present.
Preparation
References
- ↑ GB Patent 1262830 - NOVEL SYDNONIMINE DERIVATIVE
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 3.0 3.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 15.0 15.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs not assigned an ATC code
- Dopamine reuptake inhibitors
- Oxadiazoles
- Prodrugs
- Russian drugs
- Stimulants
- Ureas
- Drugs in the Soviet Union
