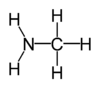
Methylamine
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
| IUPAC name
aminomethane, methanamine
|
|||
Other names
|
|||
| Identifiers | |||
| 74-89-5 |
|||
| 3DMet | B00060 | ||
| Abbreviations | MMA | ||
| 741851 | |||
| ChEBI | CHEBI:16830 |
||
| ChEMBL | ChEMBL43280 |
||
| ChemSpider | 6089 |
||
| DrugBank | DB01828 |
||
| EC Number | 200-820-0 | ||
| 145 | |||
| Jmol 3D model | Interactive image | ||
| KEGG | C00218 |
||
| MeSH | methylamine | ||
| PubChem | 6329 | ||
| RTECS number | PF6300000 | ||
| UN number | 1061 | ||
|
|||
|
|||
| Properties | |||
| CH5N | |||
| Molar mass | 31.06 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Fishy, ammoniacal | ||
| Density | 656.2 kg m−3 (at 25 °C) | ||
| Melting point | −93.10 °C; −135.58 °F; 180.05 K | ||
| Boiling point | −6.6 to −6.0 °C; 20.0 to 21.1 °F; 266.5 to 267.1 K | ||
| 1.08 kg L−1 (at 20 °C) | |||
| log P | −0.472 | ||
| Vapor pressure | 186.10 kPa (at 20 °C) | ||
|
Henry's law
constant (kH) |
1.4 mmol Pa−1 kg−1 | ||
| Basicity (pKb) | 3.36 | ||
| Viscosity | 230 μPa s (at 0 °C) | ||
| 1.31 D | |||
| Thermochemistry | |||
|
Std enthalpy of
formation (ΔfH |
−23.5 kJ mol−1 | ||
| Vapor pressure | {{{value}}} | ||
| Related compounds | |||
|
Related alkanamines
|
ethylamine, dimethylamine, trimethylamine | ||
|
Related compounds
|
ammonia | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to fish. Methylamine is used as a building block for the synthesis of many other commercially available compounds.
Contents
Industrial production
Methylamine is prepared commercially by the reaction of ammonia with methanol in the presence of a silicoaluminate catalyst. Dimethylamine and trimethylamine are coproduced; the reaction kinetics and reactant ratios determine the ratio of the three products. The product most favoured by the reaction kinetics is trimethylamine.[1]
- CH3OH + NH3 → CH3NH2 + H2O
In this way, an estimated 115,000 tons were produced In 2005.[2]
Laboratory methods
Methylamine was first prepared in 1849 by Wurtz by the hydrolysis of methyl isocyanate and related compounds.[2][3] An example of this process includes the use of Hofmann rearrangement to yield methylamine from acetamide and bromine gas.[4][5]
In the laboratory methylamine hydrochloride is readily prepared by various other methods. One method entails treating formaldehyde with ammonium chloride.[6]
- NH4Cl + H2CO → [CH2=NH2]Cl + H2O
- [CH2=NH2]Cl + H2CO + H2O → [CH3NH3]Cl + HCOOH
The colorless hydrochloride salt can be converted to the amine by the addition of strong base, like NaOH:
- [CH3NH3]Cl + NaOH → CH3NH2 + NaCl + H2O
Another method entails reducing nitromethane with zinc and hydrochloric acid.[7]
Reactivity and applications
Methylamine is a good nucleophile as it is highly basic and unhindered, although, as an amine it is considered a weak base. Its use in organic chemistry is pervasive. Some reactions involving simple reagents include: with phosgene to methyl isocyanate, with carbon disulfide and sodium hydroxide to the sodium methyldithiocarbamate, with chloroform and base to methyl isocyanide and with ethylene oxide to methylethanolamines. Liquid methylamine has solvent properties analogous to those for liquid ammonia.[8]
Representative commercially significant chemicals produced from methylamine include the pharmaceuticals ephedrine and theophylline, the pesticides carbofuran, carbaryl, and metham sodium, and the solvents N-methylformamide and N-methylpyrrolidone. The preparation of some surfactants and photographic developers require methylamine as a building block.[2]
Biological chemistry
Methylamine arises as a result of putrefaction and is a substrate for methanogenesis.[9]
Additionally, methylamine is produced during PADI4-dependent arginine demethylation.[10]
Safety
The LD50 (mouse, s.c.) is 2.5 g/kg.[11]
The Occupational Safety and Health Administration (OSHA) and National Institute for Occupational Safety and Health (NIOSH) have set occupational exposure limits at 10 ppm or 12 mg/m3 over an eight hour time-weighted average.[12]
Methylamine is also controlled as a List 1 precursor chemical by the United States Drug Enforcement Administration due to its use in the production of methamphetamine.
See also
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 2.0 2.1 2.2 Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a02_001
- ↑ Charles-Adolphe Wurtz (1849) "Sur une série d'alcalis organiques homologues avec l'ammoniaque" (On a series of homologous organic alkalis containing ammonia), Comptes rendus … , 28 : 223-226. Note: Wurtz's empirical formula for methylamine is incorrect because chemists in that era used an incorrect atomic mass for carbon (6 instead of 12).
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.; Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ M. G. DeBacker, El B. Mkadmi, F. X. Sauvage, J.-P. Lelieur, M. J. Wagner, R. Concepcion. J. Kim, L. E. H. McMills, J. L. Dye "The Lithium−Sodium−Methylamine System: Does a Low-Melting Sodide Become a Liquid Metal?" J. Am. Chem. Soc., 1996, vol. 118, pp 1997–2003. doi:10.1021/ja952634p
- ↑ Thauer, R. K., "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson", Microbiology, 1998, 144, 2377-2406.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ The Merck Index, 10th Ed. (1983), p.864, Rahway: Merck & Co.
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards
Lua error in package.lua at line 80: module 'strict' not found.

