Pyritinol
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
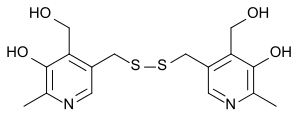
5,5'-[dithiobis(methylene)]bis[4-(hydroxymethyl)-2-methylpyridin-3-ol]
|
|
| Pharmacokinetic data | |
| Biological half-life | 2.5 hours |
| Identifiers | |
| CAS Number | 1098-97-1 |
| ATC code | N06BX02 (WHO) |
| PubChem | CID: 14190 |
| ChemSpider | 13561 |
| UNII | AK5Q5FZH2R |
| KEGG | D02160 |
| ChEMBL | CHEMBL488093 |
| Chemical data | |
| Formula | C16H20N2O4S2 |
| Molecular mass | 368.473 |
|
|
|
|
| |
|
Pyritinol also called pyridoxine disulfide or pyrithioxine (European drug names Encephabol, Encefabol, Cerbon 6) is a semi-synthetic water-soluble analog of vitamin B6 (Pyridoxine HCl). It was produced in 1961 by Merck Laboratories by bonding 2 vitamin B6 compounds (pyridoxine) together with a disulfide bridge. Since the 1970s, it has been a prescription and OTC drug in several countries for cognitive disorders and learning disorders in children. Since the early 1990s it has been sold as a nootropic dietary supplement in the United States.
It is approved for "symptomatic treatment of chronically impaired brain function in dementia syndromes" and for "supportive treatment of sequelae of craniocerebral trauma" in various European countries, including Austria, Germany, France, Italy, Portugal, and Greece.
In France it is also approved for rheumatoid arthritis as a disease modifying drug, on the basis of the results of clinical trials.
It is not licensed for use in the United Kingdom, but in many countries it is available over the counter and is widely advertised on the internet as being for "memory disturbances." From the known sales data, it is estimated that more than 100 000 individuals in European Union countries have taken pyritinol in the past five years.
One small study, with 12 subjects given pyritinol, showed an improvement in performance on tests of reaction time, but not on memory tests.[1]
Some studies have found large doses of Pyritinol can help to reduce hangovers.[2]
Adverse effects
Adverse effects include nausea, headache,[3] and rarely allergic reaction (mild skin reactions).[4] A 2004 survey of six case reports suggested a link between pyritinol and severe cholestatic hepatitis when on several drugs for certain diseases.[5]
Other rare side effects: acute pancreatitis[6] and photoallergic eruption.[7]
See also
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Unwanted Effects of Cosmetics and Drugs Used in Dermatology, Issue 282 By Anton C. de Groot, Johan Pieter Nater, J. Willem Weyland
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.