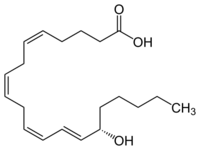
15-Hydroxyicosatetraenoic acid
 |
|
| Names | |
|---|---|
| IUPAC name
(5Z,8Z,11Z,13E,15S)-15-Hydroxyicosa-5,8,11,13-tetraenoic acid
|
|
| Other names
15-HETE, 15(S)-HETE, 15(S)-HETE
|
|
| Identifiers | |
| 54845-95-3 | |
| 3401 | |
| Jmol 3D model | Interactive image |
| PubChem | 5280724 |
|
|
| Properties | |
| C20H32O3 | |
| Molar mass | 320.47 g·mol−1 |
| Vapor pressure | {{{value}}} |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
15-Hydroxyicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an endogenous eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types and tissues first produce 15-Hydroperoxyicosatetraenoic acid (15-HpETE). These initial hydroperoxy products are extremely short lived in cells: if not otherwise metabolized, they are reduced to 15-HETE. Both these molecules are hormone-like autocrine and paracrine signalling agents, involved in inflammation response, but often are further metabolized to a wide range of products that are much more potent, including eoxins.[1][2][3]
The production and actions of these molecules and their metabolites often differ greatly depending on cell-type or tissue-type studied. In many ways they are analogous to the more abundant 5-HETE and 5-HPETE.
Contents
Nomenclature and Stereoisomers
15-HETE (15(S)-HETE, or 15S-HETE) is unambiguously designated by a shortened version of its IUPAC name as 15(S)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid. S signifies the S chirality configuration of the compound's hydroxy residue, as distinguished from its 15(R)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid, i.e. 15(R)-HETE or 15R-HETE, stereoisomer, while Z and E signify the cis–trans isomerism configurations, respectively, of its double bounds at carbon positions 5, 8, 11, and 13.
These stereoisomers are produced from corresponding stereoisomers of 15-HpETE, namely 15(S)-hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (15(S)-HpETE), 15(R)-hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (15(R)-HpETE), and/or a racemic mixture of both stereoisomers. These initial hydroperoxy products are extremely short lived in cells: if not otherwise metabolized, they are reduced to their 15(S)-HETE or 15(R)-HETE analogs.
Both stereoisomers of 15-HETE and probably also of 15-HpETE have signalling activities, as discussed below.
Production
Human tissues attack arachidonic acid with 15-lipoxygenase-1 (i.e., 15-LO-1, ALOX15) to form (15(S)-HpETE) as a major product and 12(S)-hydroperoxy-5Z,8Z,10E,15Z-eicosatetraenoic acid and 14(S),15(S)-trans-oxido-5Z,8Z,11Z-14,15-leukotriene A4 as minor products; 15(S)-hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic and 12(S)-hydroperoxy-5Z,8Z,10E,15Z-eicosatetraenoic acids, i.e. 12-hydroperoxyicosatetraenoic acid (12-HpETE), are rapidly converted to 15(S)-HETE and 12(S)-hydroxy-5Z,8Z,10E,15Z-eicosatetraenoic acid (12-Hydroxyeicosatetraenoic acid), 12(S)-HETE), respectively, or flow into numerous other metabolic pathways while 14(S),15(S)-trans-oxido-5Z,8Z,11Z-14,15-leukotriene A4 is further metabolized by 15-LO-1 to various isomers of 8,15(S)-dihydroxy-5S,8S,11Z,13S-eicosatetraenoic acids, e.g. 8,15(S)-LTB4's.[4][5][6][7][8] Human 15-LOX-2 (i.e. ALOX15B) also makes 15(S)-HpETE and 15(S)-HETE but prefers linoleic acid over arachidonic acid as substrate and therefore produces 15(S)-HpETE and 15(S)-HETE as minor products; 15-LO-2 does not make 12-HETE.[8] Human and rat microsomal cytochrome P450s, e.g. CYP2C19, metabolize arachidonic acid to a racemic mixture of 15-HETEs, i.e., 15(R,S)-HETEs, >90% of which is 15(R)-HETE.[9][10] Human prostaglandin-endoperoxide synthase 1 (COX-1) and Prostaglandin-endoperoxide synthase 2 (COX-2) metabolize arachidonic acid primarily to prostaglandins but also to small amounts of 11(R)-HETE and a racemic mixture of 15-HETEs composed of ~22% 15(R)-HETE and 78% 15(S)-HETE.[11] When pretreated with aspirin, however, COX-1 is inactive while COX-2 attacks arachidonic acid to produce almost exclusively 15(R)-HETE along with its presumed precursor 15(R)-HpETE.[11][12][13] The spontaneous and non-enzymatically-induced autooxidation of arachidonic acid yields 15(R,S)-hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic acids[14][15] which in tissues would be rapidly converted to 15(R,S)-HETEs.
Further metabolism of 15(S)-HpETE, 15(S)-HETE, 15(R)-HpETE, 15(R)-HETE, and 15-oxo-ETE
15(S)-HpETE
A) Is rapidly reduced to 15(S)-HETE by ubiquitous cellular peroxidase activities including those of prostaglandin-endoperoxide synthase,[16] prostacyclin synthase, thromboxane synthase,[17] and Glutathione peroxidases.[18]
B) Is acylated into membrane phospholipids, particularly Phosphatidylinositols[19][20] and phosphatidylethanolamine.[21][22] The 15(S)-HpETE is bound primarily at the sn-2 position of these phospholipids (see Phospholipase) and may be reduced to 15(S)-HETE[19][20][21][22] thereby forming their 15(S)-HETE-bound phospholipoid analogs. Phosphotidylinositol phospholipids with 15(S)-HETE in the sn-2 position can be attacked by phospholipase C to form corresponding diglycerides with 15(S)-HETE at theirsn-2 positions.[23]
C) Is further metabolized by 15-LO-1 to its 14,15-trans-epoxide, 14,15-trans-epoxide oxido-5Z,8Z,10E,13E-eicosatetraenoic acid (i.e., Eoxin A4 or EXA4), and thereafter to 14(R)-glutothionyl-15(S)hydroxy-5Z,8Z,10E,13E-eicosatetraenoic acid (i.e. Eoxin C4 or EXC4) by leukotriene C4 synthase.[24][25][26] EXC4 contains glutathione (i.e. γ-L-glutamyl-L-cysteinylglycine) bound in the R configuration to carbon 14. EXC4 is further metabolized by removal of the γ-L-glutamyl residue to form EXD4 which is in turn further metabolized by removal of the glycine residue to form EXE4.[27] These metabolic transformations are similar to those in the pathway that metabolizes arachidonic acid to [[LTA4]], [[LTC4]], [[LTD4]], and [[LTE4]] and presumed to be conducted by the same enzymes [24][26][28] (Eoxins are also termed 14,15-leukotrienes or 14,15-LTs).
D) is also metabolized by 15-LO-1 acid to various 8,15-diHETEs including the two 8(R) and 8(S) Diastereomers of 8,15(S)-dihydroxy-5,9,11,13-eicosatetraenoic acid (8,15-leukotrienes B4) and to two isomeric erythro-14,15-dihydroxy-5-cis-8,10,12-eicosatetraenoic acids (14,15-leukotrienes B4).[29][30][31]
E) Is further metabolized by 15-LO-1 to 11(S)-hydroxy-14(S),15(S)-epoxy-5(Z),8(Z),12(E)-eicosatrienoic acid and 13(R)-hydroxy-14(S),15(S)-epoxy-5(Z),8(Z),11(Z)-eicosatrienoic acid; these two products are novel Hepoxilins produced by ALOX15 rather than ALOX12, the enzyme responsible for making the various other hepoxilins in humans.[32] The two novel hepoxilins are termed respectively 14,15-HXA3 and 14,15-HXB3. 14,15-HXA3 can be further metabolized by glutathione transferases to 11(S),15(S)-dihydroxy-14(R)-glutathionyl--(5Z),8(Z),12(E)eicosatrienoic acid (14,15-HXA3C) which is then further metabolized to 11(S),15(S)-dihydroxy-14(R)-cysteinyl-glycyl-(5Z),8(Z),12(E)eicosatrienoic acid (14,15-HXA3D).[32]
F) Is isomerized to 15(S)-hydroxy-11,12-cis-epoxy-5Z,8Z,13E-eicosatrienoic acid (i.e., 15-H-11,12-EETA) by a hydroperoxide isomerase activity and then to 11,12,15-trihydroxy-5Z,8Z12E-eicosatrienoic acid (i.e. 11,12,15-THETA) and 11,14,15-trihydroxy-5Z,8Z,12E-eicosatrienoic acid (i.e., 11,14,15-THETA) by a soluble epoxide hydrolase activity or, alternatively, by acid in a non-enzymatic reaction(the R, S configuration of the hydroxy residues in the latter two metabolites has not been defined.[33]
G) Is isomerized to threo and erythro diastereoisomers of 13-hydroxy-14,15-cis-epoxy-5Z,8Z,11Z-eicosatrienoic acid (i.e., 15-H-11,12-EETA) by a hydroperoxide isomerase activity, possibly a Cytochrome P450, i.e. CYP2J2.[34]
H) Is metabolized in skin epidermis by Epidermis-type lipoxygenase 3 (eLOX3, encoded by the ALOXE3 gene) to make two products, hepoxilin A3 (HxA3, i.e., 13R-hydroxy-14S,15S-epoxy-5Z,8Z,11Z-eicosatetraenoic acid) and 15-oxo-ETE).[35]
I) Like other hydroperoxy-containing fatty acids, degrades in cells to various bifuctional potentially toxic electrophiles such as 4-hydroxy-2(E)-nonenal and 4-oxo-2(E)-nonenal.[36]
J) Is metabolized by cytochrome P450 (CYP) enzymes such as CYP1A1, CYP1A2, CYP1B1, and CYP2S1 to 15-oxo-ETE.[37]
15(S)-HETE
A) Is oxidized to its keto analog, 15-oxo-ETE, by the same enzyme that converts prostaglandins of the A, E, and F series to their 15-keto analogs viz., NAD+-dependent 15-hydroxyprostaglandin dehydrogenase; 15-oxo-ETE, similar to 15(S)-HETE, may be acylated into membrane phosphatidylethanolamaine [21][22] or, similar to 15(S)-HpETE, conjugated with glutathione to form a 13-cysteinyl-glycyl-glutamine adduct viz., 13-glutatione,15-oxo-5(S),8(Z),11(E)-eicosatrienoic acid; the latter metabolite is attacked by γ-glutamyl-transferase to form 13-cysteinyl-glycine,15-oxo-5(S),8(Z),11(E)-eicosatrienoic acid.[38]
B) Is acylated into membrane phospholipids, particularly phosphatidylinositol and phosphatidylethanolamine. The phospholipid products contain this 15(S)-HETE most likely at the sn-2 position; these same phospholipids may be made directly by the action of 15-LO-1 on membrane phosphatidylinositols or phosphatidylethanolamines containing arachidonic acid at the sn-2 positions.[39][40][41][42] The phosphatidylethanolamine-bound 15-HETE may be converted to phosphatidylethanolamine-bound 15-oxo-ETE.[22]
C) Is oxygenated by 5-lipoxygenase (ALOX5 to form its 5,6-trans epoxide derivative which may then rearrange to the lipoxins (LX), LXA4 (i.e. 5(S),6(R),15(S)-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid) and LXB4 (i.e., 5(S),14(R),15(S)-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid).[43][44] or to 5(S),15(S)-dihydroperoxy-6E,8Z,11Z,13E-eicosatetraenoate (i.e., (5(S),15(S)-diHETE).[45][46] 5(S),15(S)-diHETE may then be oxidized to 5-oxo-15(S)-hydroxy-6E,8Z,11Z,13E-eicosatetraenoate (i.e., 5-oxo-15(S)-hydroxy-ETE); these two metabolites may also be made by 15-LO's metabolism of 5-Hydroxyicosatetraenoic acid and 5-oxo-eicosatetraenoic acid), respectively.[47][48]
15(R)-HpETE
A) This hydroperoxy precursor of 15(R)-HETE made by COX enzymes degrades to 15(R)-HETE.[36] B) Decomposes to various bifuctional potentially toxic electrophiles such as 4-hydroxy-2(E)-nonenal and 4-oxo-2(E)-nonenal.[36]
15(R)-HETE
A) Like 15(S)-HETE is oxidized by NAD-dependent 5-hydroxyprostaglandin dehydrogenase to form 15-oxo-ETE which product can be converted its 13-cysteinyl-glycyl-glutamyl and then 13-cysteinyl-glycine products as described above for 5(S)-HETE.[38]
B) Is oxygenated by ALOX5 to form its 5,6-oxido derivative which then rearranges to the 15(R) diastereomers of LXA4 and (LXB4 viz., 15-epic LXA4 5(S),6(R),15(R)-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid) and 15-epi-LXB4 (i.e., 5(S),14(R),15(S)-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid, respectively.[43][49]
15-oxo-ETE
A) Is adducted to glutathione and thereby is preferentially exported from cells through multiple drug resistance transporters MRP1 (see ABCC10 and MRP4 (see ABCC4).[50][51]
Activities of parent metabolites and their metabolites
15(S)-HpETE and 15(S)-HETE
Most studies have analyzed the action of 15(S)-HETE but not that of its less stable precursor 15(S)-HpETE. Since this precursor is rapidly converted to 15(S)-HETE in cells, it is likely that the two metabolites share similar activities. In many studies, however, is not clear that these activities reflect their intrinsic action or reflect their further metabolism to the metabolites sited above.
15(S)-HpETE and 15(S)-HETE bind to the G protein-coupled receptor, Leukotriene B4 receptor 2, i.e. BLT2;[52] this receptor may mediate at least some of their stimulatory activities. Thus, BLT2 may be responsible in part or whole for mediating the growth-promoting and anti-apoptosis (i.e. anti-cell death) activities of 15(S)-HETE in cultured human breast cancer cells,[53] human cancer colon cells,[54] human hepatocellular HepG2 and SMMC7721 cancer cells,[55] mouse 3T3 cells (a fibroblast cell line),[56] rat PA adventitia fibroblasts,[57] Baby hamster kidney cells,[58] and diverse types of vascular endothelial cells.[59][60][61][62] These growth-stimulating effects could contribute to the progression of the cited cancer types in animal models or even humans[53][63] or to the excess fibrosis that occurs, e.g. in the narrowing of pulmonary arteries that contributes to hypoxia-induced pulmonary hypertension,[56] or accompanies liver cirrosis.[64] 15(S)-HETE may also act through BLT2 to stimulate an immediate contractile response in rat pulmonary arteries[65] and the angiogenic effect on human umbilical[60] and dermal[66] endothelial cells of the vasulature.
15(S)-HpETE and 15(S)-HETE also directly bind with and activate peroxisome proliferator-activated receptor gamma.[67] This activation may contribute to the ability of 15(S)-HETE to inhibit the growth of cultured human a) prostate cancer PC-3, LNCaP, and DU145 cells and non-malignant human prostate cells;[68][69] b) lung adenocarcinoma A549 cells;[70] c) human colorectal cancer cells;[71] d) corneal epithelial cells;[72] and e) Jurkat T-cell leukemia cells;[73] The decline in the level of 15(S)-HpETE-forming enzymes and consequential fall in cellular 15-HETE production that occurs in human prostate may be one mechanism by which this and perhaps other human cancers such as those of the colon, rectum, and lung avoid apoptosis and thereby progress and spread.[74][75] In this scenario, 15(S)-HETE and one of its formaing enzymes, particularly 15-LOX-2, appear to act as tumor suppressors.
Some of the inhibitory effects of 15(S)-HpETE and 15(S)-HETE, particularly when induced by high concentrations (e.g. >1-10 micromolar), may be due to a less specific mechanism: 15(S)-HpETE and to a lesser extent 15(S)-HETE induce the generation of Reactive oxygen species. These species trigger cells to activate their death programs, i.e. apoptosis, and/or are openly toxic to the cells.[76][77][78][79][80] 15(S)-HpETE and 15(S)-HETE inhibit angiogenesis and the growth of cultured human chronic myelogenous leukemia K-562 cells by a mechanism that is associated with the production of reactive oxygen species.[81][82][83]
Several bifuctional electrophilic breakdown products of 15(S)-HpETE, e.g. 4-hydroxy-2(E)-nonenal, 4-hydroperoxy-2(E)-nonenal, 4-oxo-2(E)-nonenal, and cis-4,5-epoxy-2(E)-decanal, are mutagens in mammalian cells and thereby may contripute to the development and/or progression of human cancers.[36]
15(R)-HETE
Similar to 15(S)-HpETE and 15(S)-HETE and with similar potency, 15(R)-HETE binds with and activates peroxisome proliferator-activated receptor gamma.[67] The precursor of 15(R)-HETE, 15(R)-HpETE may, similar to 15(S)-HpETE, break down to the mutagenic products 4-hydroxy-2(E)-nonenal, 4-hydroperoxy-2(E)-nonenal, 4-oxo-2(E)-nonenal, and cis-4,5-epoxy-2(E)-decanal and therefore be involved in cancer development and/or progression.[84]
15-oxo-ETE
In cultured human monocytes of the THP1 cell line, 15-oxo-ETE inactivates IKKβ (also known as IKK2) thereby blocking this cell's NF-κB-mediated pro-inflammatory responses (e.g.,. Lipopolysaccharide-induced production of TNFα, Interleukin 6, and IL1B) while concurrently activating anti-oxidant responses upregulated through the anti-oxidant response element (ARE) by forcing cytosolic KEAP1 to release NFE2L2 which then moves to the nucleus, binds ARE, and induces production of, e.g. hemoxygenase-1, NADPH-quinone oxidoreductase, and possibly glutamate-cysteine ligase modifier.[85] By these actions, 15-oxo-ETE may dampen inflammatory and/or Oxidative stress responses. In a cell-free system, 15-oxo-ETE is a moderately potent (IC50=1 μM) inhibitor of 12-lipoxygenase but not other human lipoxygenases.[86] This effect could also have anti-inflammatory and anti-oxidative effects by blocking the formation of 12-HETE and Hepoxilins. 15-Oxo-ETE is an example of an α,β unsaturated ketone Electrophile. These ketones are highly reactive with nucleophiles, adducting to, for example, the cysteines in transcription and transcription-related regulatory factors and enzymes to form their alkylated and thereby often inactivated products.[86][87] It is presumed that the preceding activities of 15-oxo-ETE reflect its adduction to the indicated elements.[85] 15-Oxo-ETE, at 2-10 μM, also inhibits the proliferation of cultured Human umbilical vein endothelial cells and LoVo human colorectal cancer cells [88][89] and at the extremely high concentration of 100 μM inhibits the proliferation of cultured MBA-MD-231 and MCF7 breast cancer cells as well as SKOV3 ovarian cancer cells.[90] They may use a similar "protein-adduction" mechanism; if so the target protein(s) for these effects have not been defined or even suggested. This 15-oxo-ETE action may prove to inhibit the remodeling of blood vessels and reduce the growth of the cited cell types and cancers. At sub-micromolar concentrations, 15-oxo-ETE has weak Chemotaxis activity for human monocytes and could serve to recruit this White blood cell into inflammatory responses.[91]
5-Oxo-15(S)-hydroxy-ETE
5-Oxo-15(S)-hydroxy-ETE is properly a member of the 5-HETE family of agonists which binds to the Oxoeicosanoid receptor 1, a G protein-coupled receptor, to activate its various target cells. As such, it is a potent stimulator of leukocytes, particularly eosinophils, as well as other OXE1-bearing cells including MDA-MB-231, MCF7, and SKOV3 cancer cells (see 5-Hydroxyicosatetraenoic acid and 5-oxo-eicosatetraenoic acid).[92] It also binds with and activates PPARγ and thereby can stimulate or inhibit cells independently of OXE1.[93]
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ J. Biol. Chem 256:9483-9582, 1981
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 8.0 8.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 11.0 11.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Prostaglandins 19:57-97, 1980
- ↑ Am. J. Pathol. 104:5-62, 1981
- ↑ Arch. Biochem. Biophys. 266:162-179, 1988
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 19.0 19.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 20.0 20.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 21.0 21.1 21.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 22.0 22.1 22.2 22.3 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 24.0 24.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 26.0 26.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Prostaglandins; see Eoxin). Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 32.0 32.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 36.0 36.1 36.2 36.3 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 38.0 38.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 43.0 43.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 53.0 53.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 56.0 56.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 60.0 60.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 67.0 67.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Cells. 32:1021-1027, 2011
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 85.0 85.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 86.0 86.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
External links
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Metabolic pathways
- Fatty acids
- Eicosanoids
- Cell biology
- Immunology