Idelalisib
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
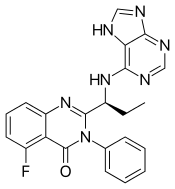
5-Fluoro-3-phenyl-2-[(1S)-1-(7H-purin-6-ylamino)propyl]-4(3H)-quinazolinone
|
|
| Clinical data | |
| Pronunciation | /aɪˈdɛləlɪˌsɪb/ eye-DEL-ə-li-sib |
| Trade names | Zydelig |
| AHFS/Drugs.com | entry |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral (tablets) |
| Pharmacokinetic data | |
| Protein binding | >84%[1] |
| Metabolism | Aldehyde oxidase (~70%), CYP3A4 (~30%);[2] UGT1A4 (minor) |
| Metabolites | GS-563117 (inactive in vitro) |
| Onset of action | Tmax = 1.5 hours |
| Biological half-life | 8.2 hours |
| Excretion | Feces (78%), urine (14%) |
| Identifiers | |
| CAS Number | 870281-82-6 |
| ATC code | L01XX47 (WHO) |
| PubChem | CID: 11625818 |
| DrugBank | DB09054 |
| ChemSpider | 9800565 |
| KEGG | D10560 |
| ChEBI | CHEBI:82701 |
| ChEMBL | CHEMBL2216870 |
| Synonyms | GS-1101, CAL-101 |
| Chemical data | |
| Formula | C22H18FN7O |
| Molecular mass | 415.42 g/mol |
|
|
|
|
Idelalisib (trade name Zydelig zye-DEL-ig,[1] codenamed GS-1101 or CAL-101) is a drug used for the treatment of certain hematological malignancies. The substance acts as a phosphoinositide 3-kinase inhibitor; more specifically, it blocks P110δ, the delta isoform of the enzyme phosphoinositide 3-kinase.[3][4]
Contents
Medical uses
Idelalisib is a second-line drug for patients whose chronic lymphocytic leukemia (CLL) has relapsed. Used in combination with rituximab,[5] idelalisib is to be used in patients for whom rituximab alone would be considered appropriate therapy due to other existing medical conditions.[5] It appears to be very effective and leads to rapid resolution of lymphadenopathy and splenomegaly. However, the lymphocyte counts takes longer to decrease to normal levels with idelalisib. Idelalisib is effective in patients who have a p53 mutation, which otherwise tends to impart a poor prognosis in CLL patients. This is important as even the first line chemotherapy regimens, such as those incorporating fludarabine are ineffective in patients with p53 mutation.
It is also approved for the treatment of follicular B-cell non-Hodgkin lymphoma (FL) and relapsed small lymphocytic lymphoma (SLL), both in patients who have received at least two prior systemic therapies.[1]
Clinical trials
It was also being studied in clinical trials for first-line treatment in high-risk CLL patients, who are otherwise unable to tolerate aggressive chemotherapy due to their medical history or age, but these trials were terminated due to adverse effects.[6]
Adverse effects
Clinical symptoms include diarrhea, fever, fatigue, nausea, cough, pneumonia, abdominal pain, chills and rash. Laboratory abnormalities may include: neutropenia, hypertriglyceridemia, hyperglycemia and elevated levels of liver enzymes. Idelalisib's safety and effectiveness to treat relapsed FL and relapsed SLL were established in a clinical trial with 123 participants with slow-growing (indolent) non-Hodgkin lymphomas. All participants were treated with idelalisib and were evaluated for complete or partial disappearance of their cancer after treatment (objective response rate, or ORR). Results showed 54% of participants with relapsed FL and 58% of participants with SLL experienced ORR.[7]
The U.S. label for idelalisib has a boxed warning describing toxicities that can be serious and fatal, including liver toxicity, severe diarrhea, colon inflammation, lung tissue inflammation (pneumonitis) and intestinal perforation, and the manufacturer was required to put in place a Risk Evaluation and Mitigation Strategy (REMS) under which the risk of toxicities would be managed.[8]
In March 2016, as reports were made from three ongoing clinical trials of serious adverse events and deaths, mostly due to infections the European Medicines Agency opened a review of the drug and its risks.[9] On March 21, 2016 Gilead Sciences (the manufacturer of idelalisib) alerted healthcare providers about decreased overall survival and increased risk of serious infections in patients with CLL and indolent non-Hodgkin lymphoma (iNHL) treated with idelalisib.[10] The company also disclosed that it stopped six clinical trials in patients with CLL, SLL and iNHL due to an increased rate of adverse events, including deaths.[11]
Pharmacology
Mechanism of action
PI3Kδ kinase is expressed in normal and malignant B-cells. By inhibiting it, idelalisib induces apoptosis and prevents proliferation in cell lines derived from malignant B-cells and in primary tumor cells. It also inhibits several cell signaling pathways, including B-cell receptor (BCR) signaling and the CXCR4 and CXCR5 signaling, which are involved in trafficking and homing of B-cells to the lymph nodes and bone marrow.[1]
Binding profile
Idelalisib is a competitive inhibitor of the ATP binding site of the PI3Kδ catalytic domain. Its in vitro potency and selectivity relative to the other Class I PI3K isoforms is the following:[12]
| PI3K isoform | IC50, nM | IC50-based PI3Kδ-fold selectivity |
|---|---|---|
| PI3Kα | 8,600 | 453 |
| PI3Kβ | 4,000 | 211 |
| PI3Kγ | 2,100 | 110 |
| PI3Kδ | 19 | 1 |
History
Regulatory
In July 2014, the FDA and EMA granted idelalisib approval to treat different types of leukemia.[7][13] The FDA is also granted approval for idelalisib to treat patients with relapsed follicular B-cell non-Hodgkin lymphoma and relapsed small lymphocytic lymphoma. Idelalisib is intended to be used in patients who have received at least two prior systemic therapies.
References
- ↑ 1.0 1.1 1.2 1.3 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 5.0 5.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Therapy Focus - TG Could Benefit From Zydelig Setback. March 2016
- ↑ 7.0 7.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
External links
- Drugs with non-standard legal status
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Antineoplastic drugs
- Breakthrough therapy
- Phosphoinositide 3-kinase inhibitors
- Purines
- Quinazolinones