Letrozole
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
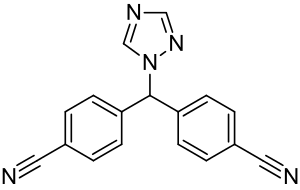
4,4'-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile
|
|
| Clinical data | |
| Trade names | Femara |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a698004 |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral |
| Pharmacokinetic data | |
| Bioavailability | 99.9% |
| Protein binding | 60%, mainly to albumin |
| Metabolism | pharmacologically-inactive carbinol metabolite (4,4΄-methanol-bisbenzonitrile)[1] |
| Biological half-life | 2 days[1] |
| Excretion | Kidneys[1] |
| Identifiers | |
| CAS Number | 112809-51-5 |
| ATC code | L02BG04 (WHO) |
| PubChem | CID: 3902 |
| IUPHAR/BPS | 5209 |
| DrugBank | DB01006 |
| ChemSpider | 3765 |
| UNII | 7LKK855W8I |
| KEGG | D00964 |
| ChEBI | CHEBI:6413 |
| ChEMBL | CHEMBL1444 |
| Chemical data | |
| Formula | C17H11N5 |
| Molecular mass | 285.303 g/mol |
|
|
|
|
| (verify) | |
Letrozole (INN, trade name Femara) is an oral non-steroidal aromatase inhibitor for the treatment of hormonally-responsive breast cancer after surgery.
Contents
Uses
FDA-approved use
Letrozole is approved by the United States Food and Drug Administration (FDA) for the treatment of local or metastatic breast cancer that is hormone receptor positive or has an unknown receptor status in postmenopausal women.[2]
Off-label uses
Letrozole has been used for ovarian stimulation by fertility doctors since 2001 because it has fewer side-effects than clomiphene (Clomid) and less chance of multiple gestation. A Canadian study presented at the American Society of Reproductive Medicine 2005 Conference suggested that letrozole may increase the risk of birth defects.[citation needed] A more detailed ovulation induction follow-up study found that letrozole, compared with a control group of clomiphene, had significantly lower congenital malformations and chromosomal abnormalities at an overall rate of 2.4% (1.2% major malformations) compared with clomiphene 4.8% (3.0% major malformations).[3] Despite this, India banned the usage of letrozole in 2011, citing potential risks to infants.[4] In 2012, an Indian parliamentary committee said that the drug controller office colluded with letrozole's makers to approve the drug for infertility in India and also stated that letrozole's use for infertility was illegal worldwide;[5] however, such off-label uses are legal in many countries such as the US and UK.[6][7]
The anti-estrogen action of letrozole has been shown to be useful in pretreatment for termination of pregnancy, in combination with misoprostol. It can be used in place of mifepristone, which is expensive and unavailable in many countries.[8]
Letrozole is sometimes used as a treatment for gynecomastia, although it is probably most effective at this if caught in an early stage (such as in users of anabolic steroids).[9][10][unreliable source?]
Some studies have shown that letrozole can be used to promote spermatogenesis in male patients suffering from nonobstructive azoospermia.[11]
Letrozole has also been shown to delay the fusing of the growth plates in mice.[12] When used in combination with growth hormone, letrozole has been shown effective in one adolescent boy with a short stature.[13]
Letrozole has also been used to treat endometriosis.[14]
Mechanism of action
Estrogens are produced by the conversion of androgens through the activity of the aromatase enzyme. Estrogens then bind to an estrogen receptor, which causes cells to divide.
Letrozole prevents the aromatase from producing estrogens by competitive, reversible binding to the heme of its cytochrome P450 unit. The action is specific, and letrozole does not reduce production of mineralo- or corticosteroids.
Contraindications
Letrozole is contraindicated in women having a pre-menopausal hormonal status, during pregnancy and lactation.[15]
Adverse effects
The most common side effects are sweating, hot flashes, arthralgia (joint pain), and fatigue.[15]
Generally, side effects include signs and symptoms of hypoestrogenism. There is concern that long term use may lead to osteoporosis,[2] which is in certain patient populations such as post-menopausal women or osteoporotics, bisphosphonates may also be prescribed.[citation needed]
Interactions
Letrozole inhibits the liver enzyme CYP2A6, and to a lesser extent CYP2C19, in vitro, but no relevant interactions with drugs like cimetidine and warfarin have been observed.[15]
Comparison with tamoxifen
Tamoxifen is also used to treat hormonally-responsive breast cancer, but it does so by interfering with the estrogen receptor. However, letrozole is effective only in post-menopausal women, in whom estrogen is produced predominantly in peripheral tissues (i.e. in adipose tissue, like that of the breast) and a number of sites in the brain.[16] In pre-menopausal women, the main source of estrogen is from the ovaries not the peripheral tissues, and letrozole is ineffective.
In the BIG 1–98 Study, of post-menopausal women with hormonally-responsive breast cancer, letrozole reduced the recurrence of cancer, but did not change survival rate, compared to tamoxifen.[17][18]
See also
- Aromatase inhibitor
- Anastrozole
- Palbociclib, doubles PFS of letrozole alone
References
- ↑ 1.0 1.1 1.2 003330 Letrozole
- ↑ 2.0 2.1 Drugs.com: monograph for letrozole. It is also used for ovarian cancer patients after they have completed chemotherapy.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Endometriosis ESHRE abstract
- ↑ 15.0 15.1 15.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer, the BIG 1–98 Collaborative Group, N Engl J Med, 361:766, 2009 Aug 20
- ↑ 32nd Annual San Antonio Breast Cancer Symposium
External links
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles with unsourced statements from April 2010
- Articles lacking reliable references from September 2012
- Articles with unsourced statements from April 2012
- Articles that show a Medicine navs template
- Aromatase inhibitors
- Hormonal antineoplastic drugs
- Triazoles
- Nitriles
