Procarbazine
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
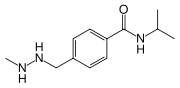
N-Isopropyl-4-[(2-methylhydrazino)methyl]benzamide
|
|
| Clinical data | |
| Trade names | Matulane |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682094 |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral (Gel Capsule), intravenous |
| Pharmacokinetic data | |
| Metabolism | Hepatic, Renal |
| Biological half-life | 10 minutes |
| Excretion | Renal |
| Identifiers | |
| CAS Number | 671-16-9 |
| ATC code | L01XB01 (WHO) |
| PubChem | CID: 4915 |
| IUPHAR/BPS | 7278 |
| DrugBank | DB01168 |
| ChemSpider | 4746 |
| UNII | 35S93Y190K |
| KEGG | D08423 |
| ChEBI | CHEBI:71417 |
| ChEMBL | CHEMBL1321 |
| Chemical data | |
| Formula | C12H19N3O |
| Molecular mass | 221.299 g/mol |
|
|
|
|
| |
|
Procarbazine (Matulane (US), Natulan (Canada), Indicarb (India) is an antineoplastic chemotherapy drug for the treatment of Hodgkin's lymphoma and certain brain cancers (such as glioblastoma multiforme).
It is a member of a group of medicines called alkylating agents. The drug is metabolized and activated in the liver. It also inhibits MAO thus increasing the effects of sympathomimetics, TCAs, and tyramine.
It gained FDA Approved in July 1969. It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[1]
Medical uses
When used to treat Hodgkin's lymphoma, it is often delivered as part of the BEACOPP regimen that includes bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine (tradename Oncovin), prednisone, and procarbazine. The first combination chemotherapy developed for Hodgkin's lymphoma (HL), MOPP also included procarbazine (ABVD has supplanted MOPP as standard first line treatment for HL, with BEACOPP as an alternative for advanced/unfavorable HL). Alternatively, when used to treat certain brain tumors (malignant gliomas), it is often dosed as PCV when combined with lomustine (often called CCNU) and vincristine.
Side effects
When combined with ethanol, procarbazine may cause a disulfiram-like reaction in some patients. It also inhibits the liver's CYP450 microsomal system, which leads to an increased effect of barbiturates, phenothiazenes, and narcotics normally metabolized by the CYP450 enzymes. Has monamine oxidase inhibition properties (MAOI), and should not be taken with most antidepressants and certain migraine medications.
Inhibits MAO in the gastrointestinal system thus can cause hypertensive crises if associated with the ingestion of tyramine-rich foods such as aged cheeses.
Procarbazine rarely causes chemotherapy-induced peripheral neuropathy,[2] a progressive, enduring, often irreversible tingling numbness, intense pain, and hypersensitivity to cold, beginning in the hands and feet and sometimes involving the arms and legs.[3]
Pharmacology
Its mechanism of action is not fully understood. Metabolism yields azo-procarbazine and hydrogen peroxide[citation needed] which results in the breaking of DNA strands.
Dose
Dose should be adjusted for renal (kidney) disease or hepatic (liver) disease.
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ del Pino BM. Chemotherapy-induced Peripheral Neuropathy. NCI Cancer Bulletin. Feb 23, 2010;7(4):6.
External links
- Drugs with non-standard legal status
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles with unsourced statements from August 2010
- Hydrazines
- DNA replication inhibitors
- IARC Group 2A carcinogens
- Monoamine oxidase inhibitors
- Benzamides
- Mutagens
- World Health Organization essential medicines