Abarelix
From Infogalactic: the planetary knowledge core
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
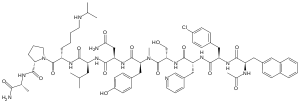
acetyl-D-β-naphthylalanyl- D-4-chlorophenylalanyl-D-3-pyridylalanyl-L-seryl-L- N-methyl- tyrosyl-D-asparagyl-L-leucyl-L-N(e )-isopropyl-lysyl-L-prolyl-D-alanyl-amide
|
|
| Clinical data | |
| Trade names | Plenaxis |
| AHFS/Drugs.com | monograph |
| Pregnancy category |
|
| Routes of administration |
Intramuscular injection |
| Pharmacokinetic data | |
| Protein binding | 96–99% |
| Identifiers | |
| CAS Number | 183552-38-7 |
| ATC code | L02BX01 (WHO) |
| PubChem | CID: 16131215 |
| IUPHAR/BPS | 1188 |
| DrugBank | DB00106 |
| ChemSpider | 10482301 |
| UNII | W486SJ5824 |
| KEGG | D02738 |
| ChEBI | CHEBI:337298 |
| ChEMBL | CHEMBL1252 |
| Chemical data | |
| Formula | C72H95ClN14O14 |
| Molecular mass | 1416.06 g/mol |
|
|
|
|
| |
|
Abarelix (trade name Plenaxis) is an injectable gonadotropin-releasing hormone antagonist (GnRH antagonist). It is primarily used in oncology to reduce the amount of testosterone made in patients with advanced symptomatic prostate cancer for which no other treatment options are available.[1][2]
It was originally marketed by Praecis Pharmaceuticals as Plenaxis,[1] and is now marketed by Speciality European Pharma in Germany[3] after receiving a marketing authorisation in 2005.