Cytarabine
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
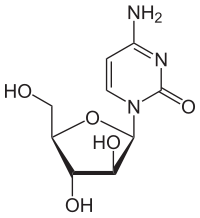
4-amino-1-[(2R,3S,4R,5R)-3,4-dihydroxy-5- (hydroxymethyl)oxolan-2-yl] pyrimidin-2-one
|
|
| Clinical data | |
| Trade names | Cytosar-U |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682222 |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Injectable (intravenous injection or infusion, intrathecal, or subcutaneously) |
| Pharmacokinetic data | |
| Bioavailability | 20% oral |
| Protein binding | 13% |
| Metabolism | Liver |
| Biological half-life | biphasic: 10 min, 1-3 hr |
| Excretion | Renal |
| Identifiers | |
| CAS Number | 147-94-4 |
| ATC code | L01BC01 (WHO) |
| PubChem | CID: 6253 |
| IUPHAR/BPS | 4827 |
| DrugBank | DB00987 |
| ChemSpider | 6017 |
| UNII | 04079A1RDZ |
| KEGG | D00168 |
| ChEBI | CHEBI:28680 |
| ChEMBL | CHEMBL803 |
| Chemical data | |
| Formula | C9H13N3O5 |
| Molecular mass | 243.217 g/mol |
|
|
|
|
| (verify) | |
Cytarabine or cytosine arabinoside (Cytosar-U or Depocyt) is a chemotherapy agent used mainly in the treatment of cancers of white blood cells such as acute myeloid leukemia (AML) and non-Hodgkin lymphoma.[1] It is also known as ara-C (arabinofuranosyl cytidine).[2] It kills cancer cells by interfering with DNA synthesis.
It is called cytosine arabinoside because it combines a cytosine base with an arabinose sugar. Cytosine normally combines with a different sugar, deoxyribose, to form deoxycytidine, a component of DNA. Certain sponges, where it was originally found, use arabinoside sugars to form a different compound (not part of DNA). Cytosine arabinoside is similar enough to human cytosine deoxyribose (deoxycytidine) to be incorporated into human DNA, but different enough that it kills the cell. This mechanism is used to kill cancer cells. Cytarabine is the first of a series of cancer drugs that altered the sugar component of nucleosides. Other cancer drugs modify the base.[3]
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[4]
Contents
Medical uses
Cytarabine is mainly used in the treatment of acute myeloid leukaemia, acute lymphocytic leukaemia (ALL) and in lymphomas,[5] where it is the backbone of induction chemotherapy.
Cytarabine also possesses antiviral activity, and it has been used for the treatment of generalised herpesvirus infection. However, cytarabine is not very selective in this setting and causes bone marrow suppression and other severe side effects. Therefore, ara-C is not a useful antiviral agent in humans because of its toxic profile[6] and actually it is used mainly for the chemotherapy of hematologic cancers.
Cytarabine is also used in the study of the nervous system to control the proliferation of glial cells in cultures, the amount of glial cells having an important impact on neurons.[citation needed]
Side effects
One of the unique toxicities of cytarabine is cerebellar toxicity when given in high doses, which may lead to ataxia. Cytarabine may cause granulocytopenia and other impaired body defenses, which may lead to infection, and thrombocytopenia, which may lead to hemorrhage.
Toxicity: leukopenia, thrombocytopenia, anemia, GI disturbances, stomatitis, conjunctivitis, pneumonitis, fever, and dermatitis, palmar-plantar erythrodysesthesia. Rarely, myelopathy has been reported after high dose or frequent intrathecal Ara-C administration.[7]
To prevent the side effects and improve the therapeutic efficiency, various derivatives of these drugs (including amino acid, peptide, fatty acid and phosphates) have been evaluated, as well as different delivery systems.[8]
Mechanism of action
Cytosine arabinoside interferes with the synthesis of DNA. It is an antimetabolic agent with the chemical name of 1β-arabinofuranosylcytosine. Its mode of action is due to its rapid conversion into cytosine arabinoside triphosphate, which damages DNA when the cell cycle holds in the S phase (synthesis of DNA). Rapidly dividing cells, which require DNA replication for mitosis, are therefore most affected. Cytosine arabinoside also inhibits both DNA[9] and RNA polymerases and nucleotide reductase enzymes needed for DNA synthesis.
When used as an antiviral, cytarabine functions by inhibiting deoxycytidine use.[10]
Cytarabine is rapidly deaminated in the body into the inactive uracil derivative and therefore is often given by continuous intravenous infusion.
History
Cytarabine was first synthesized in 1959 by Richard Walwick, Walden Roberts, and Charles Dekker at the University of California, Berkeley.[11]
It was approved by the United States Food and Drug Administration in June 1969, and was initially marketed in the U.S. by Upjohn under the trade name Cytosar-U.
Brand names
- Cytosar-U
- Tarabine PFS (Pfizer)
- Depocyt (longer-lasting liposomal formulation)
- AraC
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.[dead link]
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
External links
- MedlinePlus page on cytarabine
- ADAP drugs page on cytarabine
- BC Cancer network page on cytarabine
- Chembank entry
- Sea to Ara C An essay on the history of cytarabine.
- Articles with dead external links from July 2010
- Drugs with non-standard legal status
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles with unsourced statements from February 2013
- Pyrimidine antagonists
- Nucleosides
- Pyrimidones
- Arabinosides
- World Health Organization essential medicines