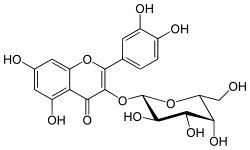
Hyperoside
 |
| Names |
| IUPAC name
2-(3,4-dihydroxyphenyl)-3-[(3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4H-chromene-4,5,7-triol
|
| Other names
Hyperozide
Hyperasid
Hyperosid
Hyperin
quercetin galactoside
Quercetin-3-galactoside
Quercetin-3-O-galactoside
|
| Identifiers |
|
|
482-36-0  Y Y |
| ChEBI |
CHEBI:67486  N N |
| ChemSpider |
4444962  N N |
| Jmol 3D model |
Interactive image |
| PubChem |
5281643 |
-
InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-27,29-30H,6H2/t13-,15+,17+,18-,21+/m1/s1  N NKey: OVSQVDMCBVZWGM-DTGCRPNFSA-N  N N
-
InChI=1/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-27,29-30H,6H2/t13-,15+,17+,18-,21+/m1/s1
Key: OVSQVDMCBVZWGM-DTGCRPNFBG
|
-
c1cc(c(cc1c2c(c(=O)c3c(cc(cc3o2)O)O)O[C@H]4[C@@H]([C@H]([C@H]([C@H](O4)CO)O)O)O)O)O
|
| Properties |
|
|
C21H20O12 |
| Molar mass |
464.38 g·mol−1 |
| Density |
1.879 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
 N verify (what is N verify (what is  Y Y N ?) N ?) |
| Infobox references |
|
|
Hyperoside is a chemical compound. It is the 3-O-galactoside of quercetin.
Natural occurrences
Hyperoside has been isolated from Drosera rotundifolia, from the Lamiaceae Stachys sp. and Prunella vulgaris, from Rumex acetosella, Cuscuta chinensis seeds, from St John’s wort and from Camptotheca acuminata.[1] It is one of the phenolic compounds in the invasive plant Carpobrotus edulis and contributes to the antibacterial[2] and antioxidant[3] properties of the plant.
In Rheum nobile and R. rhaponticum, it serves as a UV blocker found in the bracts.
It is also found in Geranium niveum[4] and Taxillus kaempferi.[5]
Uses and actions
It can have a protective antioxidant effect on cultured PC12 cells.[6]
Like various other flavonoids, hyperoside has been found to possess antagonist activity at the κ-opioid receptor.[7]
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of Taxillus kaempferi. Konishi T, Nishio T, Kiyosawa S, Fujiwara Y and Konoshima T, Yakugaku Zasshi., February 1996, volume 116, issue 2, pages 148-157 (article in Japanese)
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
|
|
Receptor
(ligands) |
| MOR |
|
| DOR |
|
| KOR |
- Agonists: 6'-GNTI
- 8-CAC
- 18-MC
- 14-Methoxymetopon
- β-Chlornaltrexamine
- β-Funaltrexamine
- Adrenorphin (metorphamide)
- Akuuamicine
- Alazocine
- Allomatrine
- Asimadoline
- BAM-12P
- BAM-18P
- BAM-22P
- Big dynorphin
- Bremazocine
- BRL-52537
- Butorphan
- Butorphanol
- BW-373U86
- Cebranopadol
- Ciprefadol
- CR665
- Cyclazocine
- Cyclorphan
- Cyprenorphine
- Diamorphine (heroin)
- Diacetylnalorphine
- Difelikefalin
- Dihydroetorphine
- Dihydromorphine
- Diprenorphine
- Dynorphin A
- Dynorphin B (rimorphin)
- Eluxadoline
- Enadoline
- Eptazocine
- Erinacine E
- Ethylketazocine
- Etorphine
- Fedotozine
- Fentanyl
- Gemazocine
- GR-89696
- GR-103545
- Hemorphin-4
- Herkinorin
- HS665
- Hydromorphone
- HZ-2
- Ibogaine
- ICI-199,441
- ICI-204,448
- Ketamine
- Ketazocine
- Laudanosine
- Leumorphin (dynorphin B-29)
- Levallorphan
- Levomethorphan
- Levorphanol
- Lexanopadol
- Lofentanil
- LPK-26
- Lufuradom
- Matrine
- MB-1C-OH
- Menthol
- Metazocine
- Metkefamide
- Mianserin
- Mirtazapine
- Morphine
- Moxazocine
- MR-2034
- N-MPPP
- Nalbuphine
- Nalbuphine sebacate
- NalBzOH
- Nalfurafine
- Nalmefene
- Nalodeine (N-allylnorcodeine)
- Nalorphine
- Naltriben
- Niravoline
- Norbuprenorphine
- Norbuprenorphine-3-glucuronide
- Noribogaine
- Norketamine
- O-Desmethyltramadol
- Oripavine
- Oxilorphan
- Oxycodone
- Pentazocine
- Pethidine (meperidine)
- Phenazocine
- Proxorphan
- Racemethorphan
- Racemorphan
- RB-64
- Salvinorin A (salvia)
- Salvinorin B ethoxymethyl ether
- Salvinorin B methoxymethyl ether
- Samidorphan
- SKF-10047
- Spiradoline (U-62,066)
- TH-030418
- Thienorphine
- Tifluadom
- Tricyclic antidepressants (e.g., amitriptyline, desipramine, imipramine, nortriptyline)
- U-50,488
- U-54,494A
- U-69,593
- Xorphanol
|
| NOP |
|
Unsorted /
unknown |
|
|
Enzyme
(inhibitors) |
|
| Others |
- Others: Kyotorphin (met-enkephalin releaser/degradation stabilizer)
|
|
|
|
Flavonols and their conjugates
|
| Backbone |
|
| Flavonols |
|
| O-Methylated flavonols |
|
| Derivative flavonols |
|
| Pyranoflavonols |
|
| Furanoflavonols |
|
| Semisynthetic |
|
