Deltorphin I
From Infogalactic: the planetary knowledge core
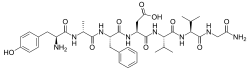 |
|
| Names | |
|---|---|
| IUPAC names
(3S)-3-[(2S)-2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]propanamido]-3-phenylpropanamido]-3-{[(1S)-1-{[(1S)-1-[(carbamoylmethyl)carbamoyl]-2-methylpropyl]carbamoyl}-2-methylpropyl]carbamoyl}propanoic acid
or L-tyrosyl-D-alanyl-L-phenylalanyl-L-α-aspartyl-L-valyl-L-valylglycinamide |
|
| Other names
[D-Ala2]Deltorphin I; Deltorphin C
|
|
| Identifiers | |
| 122752-15-2 | |
| ChEMBL | ChEMBL317956 |
| ChemSpider | 8231517 |
| Jmol 3D model | Interactive image |
| PubChem | 10055958 |
|
|
|
|
| Properties | |
| C37H52N8O10 | |
| Molar mass | 768.856 g/mol |
| Vapor pressure | {{{value}}} |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Deltorphin I, also known as [D-Ala2]deltorphin I or deltorphin C, is a naturally-occurring, exogenous opioid heptapeptide and hence, exorphin, with the amino acid sequence Tyr-D-Ala-Phe-Asp-Val-Val-Gly-NH2.[1][2] While not known to be endogenous to humans or other mammals, deltorphin I, along with the other deltorphins and the dermorphins, is produced naturally in the skin of species of Phyllomedusa,[1] a genus of frogs native to South and Central America. Deltorphin possesses very high affinity and selectivity as an agonist for the δ-opioid receptor,[1][2] and on account of its unusually high blood-brain-barrier penetration rate,[3] produces centrally-mediated analgesic effects in animals even when administered peripherally.[4]