Methasterone
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
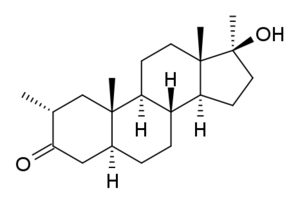
17β-Hydroxy-2α,17α-dimethyl-5α-androstane-3-one
|
|
| Clinical data | |
| Legal status |
|
| Routes of administration |
Oral |
| Pharmacokinetic data | |
| Bioavailability | ~50% |
| Metabolism | Hepatic |
| Biological half-life | 8-12 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number | 3381-88-2 |
| ATC code | none |
| PubChem | CID: 237186 |
| ChemSpider | 207079 |
| Synonyms | Superdrol, methyldrostanolone, methasteron, drostanolone. |
| Chemical data | |
| Formula | C21H34O2 |
| Molecular mass | 318.492 g/mol |
|
|
|
|
| |
|
Methasterone (Superdrol, methasteron, and methyldrostanolone) is an oral anabolic steroid that was never marketed through legitimate channels for medicinal purposes. It was brought to market, instead, in a clandestine fashion as a “designer steroid.”
Contents
History
The synthesis of methasterone is first mentioned in the literature in 1956 in connection with research conducted by Syntex Corporation in order to discover a compound with anti-tumor properties.[1] In a 1959 research journal article, it is initially mentioned and is elaborated upon where its method of synthesis is discussed in greater detail, its tumor inhibiting properties are verified, and it is noted as being a “potent orally active anabolic agent exhibiting only weak androgenic activity.”[2] The results of subsequent assays to determine methasterone’s anabolic and androgenic activity were published in Vida’s Androgens and Anabolic Agents, a dated but still standard reference, where it was noted that methasterone possessed the oral bioavailability of methyl-testosterone while being 400% as anabolic and 20% as androgenic, yielding a Q-ratio (also known as an anabolic to androgenic ratio) of 20, which is considered very high.[3]
Injectable counterpart
Methasterone was never a commercially available prescription drug. Its non-17α-alkylated counterpart, drostanolone, was commercialized by Syntex Corporation under the brand name Masteron.[4]
"Designer steroid"
Methasterone resurfaced in 2005 as a “designer steroid”.[5] It was brought to market by Designer Supplements as the primary ingredient of a dietary supplement named Superdrol. Its introduction into commerce may have represented an attempted circumvention of the U.S. Anabolic Steroids Control Act of 1990 (along with its 2004 revision), since the law is, in part, drug-specific;[6] methasterone, as is the case with many designer steroids, was not declared a Schedule III class anabolic steroid in that act because it was not commercially available at the time the act, and its subsequent revision, were signed into law.[7] Methasterone was therefore being sold as an over-the-counter dietary supplement.
Controversy and FDA involvement
It was in late 2005 that the status of methasterone, in addition to that of four other designer steroids, as an anabolic steroid was brought to public awareness by an article published in the Washington Post.[8] Don Catlin of the UCLA Olympic Laboratory, who conducted the studies, noted methasterone’s similarity to drostanolone. A warning by the FDA was issued soon after to the general public as well as to the distributor, Designer Supplements LLC, for the marketing of this compound.[9] Methasterone was subsequently added to the World Anti-Doping Agency (WADA) list of prohibited substances in sport.[10] Despite all of this, methasterone has resurfaced within the supplement industry on several occasions since its banning by WADA.[11]
Hepatoxicity
Methasterone is hepatotoxic (toxic to the liver). Many cases of liver damage due to the use of methasterone have been cited in the medical literature.[12][13][14][15]
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- Drugs with non-standard legal status
- Chemical articles having calculated molecular weight overwritten
- Articles with changed InChI identifier
- Infobox drug articles without a structure image
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs not assigned an ATC code
- Anabolic steroids
- Designer drugs