Enzalutamide
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
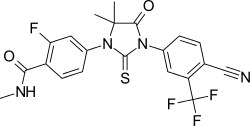
4-(3-(4-Cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide
|
|
| Clinical data | |
| Trade names | Xtandi |
| AHFS/Drugs.com | entry |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral |
| Pharmacokinetic data | |
| Biological half-life | 8–9 days[1] |
| Identifiers | |
| CAS Number | 915087-33-1 |
| ATC code | L02BB04 (WHO) |
| PubChem | CID: 15951529 |
| IUPHAR/BPS | 6812 |
| ChemSpider | 13093347 |
| UNII | 93T0T9GKNU |
| ChEBI | CHEBI:68534 |
| Chemical data | |
| Formula | C21H16F4N4O2S |
| Molecular mass | 464.44 g·mol−1 |
|
|
|
|
Enzalutamide (INN, USAN) (brand name Xtandi and former developmental code name MDV3100) is a synthetic, non-steroidal pure antiandrogen which was developed by the pharmaceutical company Medivation for the treatment of metastatic castration-resistant prostate cancer.[2] Medivation has reported up to an 89% decrease in serum prostate specific antigen (PSA) levels after a month of taking the drug.[3] Research suggests that enzalutamide may also be effective in the treatment of certain types of breast cancer.[4][5] In August 2012, the United States (U.S.) Food and Drug Administration (FDA) approved enzalutamide for the treatment of castration-resistant prostate cancer.[6][7]
Contents
Discovery
Enzalutamide was discovered by Charles Sawyers who is now at Memorial Sloan–Kettering Cancer Center and Michael Jung at UCLA.[8][9]
Preclinical pharmacology
Enzalutamide has approximately 5- to 8-fold higher binding affinity for the androgen receptor (AR) compared to bicalutamide.[10][11] As opposed to bicalutamide, enzalutamide does not promote translocation of AR to the cell nucleus and in addition prevents binding of AR to deoxyribonucleic acid (DNA) and AR to coactivator proteins.[10] As such, it has been described as an AR signaling inhibitor in addition to antagonist.[12]
When LNCaP cells (a prostate cancer cell line) engineered to express elevated levels of AR (as found in patients with advanced prostate cancer) were treated with enzalutamide, the expression of androgen-dependent genes PSA and TMPRSS2 was down regulated in contrast to bicalutamide where the expression was upregulated.[10] In VCaP cells which over-express the AR, enzalutamide induced apoptosis whereas bicalutamide did not.[10] Furthermore, enzalutamide behaves as an antagonist of the W741C mutant AR in contrast to bicalutamide which behaves as a pure agonist when bound to the W741C mutant.[10]
Clinical studies
Enzalutamide is clinically-active in metastatic castration-resistant prostate cancer.[13] PSA level decreased more than 50% in 40/65 chemo-naive patients and 38/75 chemotherapy-treated patients.[13] Median time to radiographic progression was 56 weeks for chemo-naive patients and 25 weeks for the post-chemotherapy population.[14]
Medivation conducted an international phase III trial that began in September 2009 known as AFFIRM. The aim of this trial was determine the safety and effectiveness of enzalutamide in patients who have previously failed chemotherapy treatment with docetaxel.[15] In November 2011, this trial was stopped early after an interim analysis revealed that patients given the drug lived for approximately 5 months longer than those taking placebo.[16] FDA approval was granted in August 2012.[6][17]
Another phase III trial known as PREVAIL is investigating the effectiveness of enzalutamide with patients who have not yet received chemotherapy.[18] On October 22, 2011, Medivation and Astellas announced that the PREVAIL trial met both co-primary endpoints of overall survival, with a 30% reduction in the risk of death compared with placebo (hazard ratio = 0.7; 95% confidence interval, range of 0.59-0.83), and radiographic progression-free survival, with an 81% reduction in risk of radiographic progression or death compared with placebo (hazard ratio = 0.19); 95% conficence interval, 0.15-0.23).[19] In addition, a phase II trial began in March 2011 comparing enzalutamide with bicalutamide in prostate cancer patients who have progressed while on gonadotropin-releasing hormone (GnRH) analogue therapy (e,g., leuprorelin) or surgical castration.[20][21]
Enzalutamide, at a dosage of 160 mg/day, has been found to produce similar increases in testosterone, estradiol, and luteinizing hormone (LH) levels relative to high-dosage bicalutamide (300–600 mg/day), and substantially greater increases in testosterone levels relative to 150 mg/day bicalutamide (114% versus 66%), suggesting that, in accordance with its higher binding affinity to the AR and AR-related mechanistic improvements, enzalutamide is a significantly more potent and effective antiandrogen in comparison.[22][23]
Adverse effects
Notable side effects of enzalutamide seen in clinical trials have included gynecomastia, breast pain/tenderness, fatigue, diarrhea, hot flashes, headache, sexual dysfunction, and, less commonly, seizures.[12][24][25][26]
Enzalutamide is regarded as having a moderate negative effect on sexual function and activity, significantly less than that of GnRH analogues but similar to that of other non-steroidal antiandrogens such as bicalutamide.[22]
In regards to seizures, they have occurred in approximately 1% of patients treated with enzalutamide in clinical trials.[12][25] This is thought to be due to enzalutamide crossing the blood-brain-barrier[27][28] and exerting off-target binding to and inhibition of the GABAA receptor in the central nervous system (it has been found to inhibit the GABAA receptor in vitro[28][29] and induces seizures in animals at high doses).[12][25] In addition to seizures, other potentially GABAA receptor-related side effects observed with enzalutamide treatment in clinical trials have included anxiety, insomnia, vertigo, paresthesia, and headache.[1] Due to its ability to lower the seizure threshold, patients with known seizure disorders or brain injury should be closely monitored during enzalutamide treatment.[30]
There is a single case report of posterior reversible encephalopathy syndrome (PRES) with enzalutamide treatment.[31]
Pharmacokinetics
Enzalutamide has a very long half-life of 8–9 days.[1]
Enzalutamide is reported to be a strong inducer of the enzyme CYP3A4 and a moderate inducer of CYP2C9 and CYP2C19.[32]
Research
In September 2015, it was announced that enzalutamide had shown effectiveness in the treatment of triple-negative, AR-positive breast cancer in a phase II clinical trial.[33][34]
See also
References
- ↑ 1.0 1.1 1.2 1.3 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 6.0 6.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 10.0 10.1 10.2 10.3 10.4 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 12.0 12.1 12.2 12.3 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 13.0 13.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 22.0 22.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 25.0 25.1 25.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 28.0 28.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- Infobox drug articles without a structure image
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles containing unverified chemical infoboxes
- Articles that show a Medicine navs template
- Antiandrogens
- Benzamides
- GABAA receptor negative allosteric modulators
- Hormonal antineoplastic drugs
- Imidazolidines
- Lactams
- Nitriles
- Organofluorides
- Prostate cancer
- Thioureas