Trenbolone
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
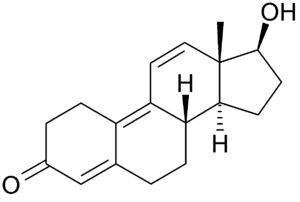
17β-Hydroxyestra-4,9,11-trien-3-one
|
|
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Intramuscular |
| Pharmacokinetic data | |
| Bioavailability | 100% (intramuscular) |
| Metabolism | ? |
| Biological half-life | 48–72 hours |
| Excretion | Urinary |
| Identifiers | |
| CAS Number | 10161-33-8 |
| ATC code | none |
| PubChem | CID: 25015 |
| ChemSpider | 23383 |
| UNII | P53R4420TR |
| Synonyms | Trienolone |
| Chemical data | |
| Formula | C18H22O2 |
| Molecular mass | 270.37 |
|
|
|
|
| |
|
Trenbolone,[pronunciation?] also known as trienolone or trienbolone,[1][2] is a steroid used on livestock to increase muscle growth and appetite. To increase its effective half-life, trenbolone is administered as a prodrug as an ester conjugate such as trenbolone acetate, trenbolone enanthate, or trenbolone cyclohexylmethylcarbonate (Parabolan). Plasma lipases then cleave the ester group in the bloodstream leaving free trenbolone.
Legal status
Bodybuilders and athletes have been known to use the drug illicitly because they experience an increase in body mass more effectively than by weight training alone and because of its reputation as a fat-burning, as well as bulking agent. In the United States, possession or use of trenbolone for humans is a violation of federal law. The DEA classifies trenbolone as a schedule III drug.[3] Trenbolone is classified as a Schedule 4 drug in Canada[4] and a class C drug with no penalty for personal use or possession in the United Kingdom.[5] Use or possession of steroids without a prescription is a crime in Australia.[6]
Use and effects
Trenbolone acetate is often referred to as "Fina" by users, because injectable trenbolone acetate was originally adapted for use by bodybuilders from dissolution of Finaplix H pellets, an ear implant used by cattle ranchers to maintain the weight of cattle during shipping to slaughter. Trenbolone improves muscle mass, feed efficiency, and mineral absorption in cattle.[7]
Trenbolone compounds have a binding affinity for the androgen receptor five times as high as that of testosterone.[8] Once metabolized, the drugs have the effect of increasing ammonium ion uptake by muscles, leading to an increase in the rate of protein synthesis. It may also have the secondary effects of stimulating appetite and decreasing the rate of catabolism, as all anabolic steroids are believed to; however, catabolism likely increases significantly once the steroid is no longer taken.[9] Trenbolone has proven popular with anabolic steroid users, as some believe it is not metabolized by aromatase or 5α-reductase into estrogenic compounds such as estradiol, or into dihydrotestosterone; however, studies on this are mixed, with some studies showing a potential increase in both.[10][11] At least one study in rats has shown trenbolone to cause gene expression with the androgen receptor at least as potent as DHT. This evidence tends to indicate Trenbolone can cause an increase in male secondary sex characteristics without the need to convert to dihydrotestosterone.[12]
Since steroids generally cause virilization effects in women in even small doses, this drug should not be taken by women. Kidney toxicity has been suggested, but has not yet been proven, and scientific evidence supporting the idea is absent from the bodybuilding community that perpetuates this idea. The origin of this myth most likely has to do with the rust-colored oxidized metabolites of trenbolone which are excreted in urine and often mistaken for blood.[13] Trenbolone and 17epi-trenbolone are both excreted in urine as conjugates that can be hydrolyzed with beta-glucuronidase.[14] This implies that trenbolone leaves the body as beta-glucuronides or sulfates.
Side effects
As with most other drugs the side effects are dose dependent, and can include the following:
- Dramatic increases in strength, heart rate and blood pressure.
- Decrease in sex drive.
- Heavy breathing (feeling of breathlessness).
- Elevated body temperature and night sweats.
- Production of dark colored urine.
- Insomnia and nightmares.
- Reduction in appetite.
- Paranoia.
- Enuresis.
Pharmacology
Trenbolone binds to and activates the androgen receptor.[2][15] It has both anabolic and sufficiently androgenic effects.[2] Trenbolone also binds with high affinity to the progesterone receptor,[2][15][16][17] and a variety of trenbolone derivatives, including altrenogest (allyl-trenbolone), norgestrienone (17α-ethynyltrenbolone), and gestrinone (ethylnorgestrienone), are used as progestins. Trenbolone binds to the glucocorticoid receptor as well.[16]
See also
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 2.0 2.1 2.2 2.3 Lua error in package.lua at line 80: module 'strict' not found. Cite error: Invalid
<ref>tag; name "Llewellyn2011" defined multiple times with different content - ↑ http://www.justice.gov/dea/pubs/scheduling.html accessed August 22, 2011.
- ↑ http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-24.html?term=steroids#sched4
- ↑ http://www.homeoffice.gov.uk/publications/alcohol-drugs/drugs/acmd1/anabolic-steroids-report/anabolic-steroids?view=Binary.
- ↑ http://www.aic.gov.au/en/crime_types/drugs_alcohol/drug_types/steroids.aspx
- ↑ http://www.depts.ttu.edu/afs/implantdb/dbhome/Revalor%20Tech%20Bulletin%2012.pdf accessed August 22, 2011.[full citation needed]
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ http://www.sportsci.org/encyc/anabster/anabster.html[full citation needed]
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ http://knol.google.com/k/dane-fletcher/trenbolone-acetate-vital-information/1yijtt1e6h32o/55#[full citation needed]
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 15.0 15.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 16.0 16.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- Pages with reference errors
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs not assigned an ATC code
- Articles needing pronunciation
- Anabolic steroids
- Androgens
- Progestogens