Amineptine
 |
|

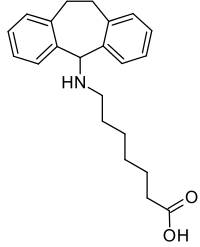
Amineptine chemical structure
|
|
| Systematic (IUPAC) name | |
|---|---|
|
7-[(10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5-yl)amino]heptanoic acid
|
|
| Clinical data | |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Biological half-life | 48 mins (original drug) 2.5 hours (metabolites)[2] |
| Excretion | Renal |
| Identifiers | |
| CAS Number | 57574-09-1 |
| ATC code | N06AA19 (WHO) |
| PubChem | CID: 34870 |
| DrugBank | DB04836 |
| ChemSpider | 32091 |
| UNII | 27T1I13L6G |
| KEGG | D07335 |
| ChEBI | CHEBI:32499 |
| ChEMBL | CHEMBL418995 |
| Chemical data | |
| Formula | C22H28NO2 |
| Molecular mass | 338.4653 g/mol |
|
|
|
|
| (verify) | |
Amineptine was developed by the French Society of Medical research in the 1960s.[3] Under the trade-names (Survector, Maneon, Directim, Neolior, Provector, Viaspera) amineptine was used as an atypical tricyclic antidepressant (TCA) that selectively inhibits the reuptake of dopamine[4] and to a lesser extent norepinephrine, in turn producing an antidepressant effect.[5]
Introduced in France in 1978 by the pharmaceutical company Servier[6] and marketed under the trade name Survector, amineptine soon gained a reputation for abuse due to its short-lived, but pleasant, stimulant effect experienced by some patients. (This is to be distinguished from its antidepressant effect, which appears in approximately seven days after commencing treatment.)
After its release into the European market, cases of hepatotoxicity emerged, some serious. This, along with the potential for abuse, led to the suspension of the French marketing authorization for Survector in 1999.[7]
Amineptine was never approved by the U.S. Food and Drug Administration (FDA) for marketing in the United States, meaning that it is not legal to market or sell amineptine for any medical uses in the US.
Contents
Therapeutic indications
Approved
Amineptine was approved in France for severe clinical depression of endogenous origin in 1978.[8]
Unapproved/off-label/investigational
Parkinson's Disease, amotivational syndromes, ADHD (Attention Deficit Hyperactivity Disorder)
Mechanism of action
- Reuptake inhibitor of dopamine and, to a lesser extent, norepinephrine.
- Very weak muscarinic and histaminic receptor antagonist.
Side effects
Dermatological
Severe acne due to amineptine was first reported in 1988 by various authors—Grupper, Thioly-Bensoussan, Vexiau, Fiet, Puissant, Gourmel, Teillac, Levigne, to name a few—simultaneously[9][10][11][12][13] in the same issue of Annales de dermatologie et de vénéréologie and in the 12 March 1988 issue of The Lancet.[14] A year later, Dr Martin-Ortega and colleagues in Barcelona, Spain reported a case of "acneiform eruption" in a 54-year-old woman whose intake of amineptine was described as "excessive."[15] One year after that, Vexiau and colleagues reported six women, one of whom never admitted to using amineptine, getting severe acne concentrated in the face, back and thorax, the severity of which varied with the dosage.[16] Most of them were treated unsuccessfully with isotretinoin (Accutane) for about 18 months; two of the three that discontinued amineptine experienced a reduction in cutaneous symptoms, with the least affected patient going into remission.[16]
Psychiatric
Psychomotor excitation can very rarely occur with this drug.
- Insomnia
- Irritability
- Nervousness
- Suicidal ideation. Seen early in the treatment, by lifting of psychomotor inhibition. This is a common occurrence with most, if not all, antidepressants.
Cardiovascular
Very rarely:
Hepatic
Amineptine can rarely cause hepatitis, of the cytolytic, cholestatic varieties.[17] Amineptine-induced hepatitis, which is sometimes preceded by a rash, is believed to be due to an allergic reaction.[18] It resolves upon discontinuation of the offending drug.[17] The risk of getting this may or may not be genetically determined.[19]
Additionally, amineptine is known to rarely elevate transaminases, alkaline phosphatase, and bilirubin.[20]
Mixed hepatitis, which is very rare, generally occurs between the 15th and 30th day of treatment. Often preceded by sometimes intense abdominal pains, nausea, vomiting or a rash, the jaundice is variable. Hepatitis is either of mixed type or with cholestatic prevalence. The evolution was, in all the cases, favorable to the discontinuation of the drug. The mechanism is discussed (immunoallergic and/or toxic).[21]
In circa 1994 Spain, there was a case associating acute pancreatitis and mixed hepatitis, after three weeks of treatment.[22]
Lazaros and colleagues at the Western Attica General Hospital in Athens, Greece reported two cases of drug induced hepatitis 18 and 15 days of treatment.[23]
One case of cytolytic hepatitis occurred after ingestion of only one tablet.[24]
Gastrointestinal
- Acute pancreatitis (very rare) A case associating acute pancreatitis and mixed hepatitis after three weeks of treatment.[22]
Immunological
In 1989, Sgro and colleagues at the Centre de Pharmacovigilance[25] in Dijon reported a case of anaphylactic shock in a woman who had been taking amineptine.[26]
Withdrawal
Pharmacodependence is very common with amineptine compared to other antidepressants.[27] A variety of psychological symptoms can occur during withdrawal from amineptine,[28] such as anxiety and agitation.[29]
Effects on the fetus
- Lacking information in humans
- Non-teratogenic in rodents
Abuse and dependence
The risk of addiction is low, but exists nonetheless. Between 1978 and 1988, there were 186 cases of amineptine addiction reported to the French Regional Centres of Pharmacovigilance; an analysis of 155 of those cases found that they were predominantly female, and that two-thirds of cases had known risk factors for addiction.[30] However, a 1981 study of known opiate addicts and schizophrenia patients found no drug addiction in any of the subjects.[31] In a 1990 study of eight amineptine dependence cases, the gradual withdrawal of amineptine could be achieved without problems in six people; in two others, anxiety, psychomotor agitation, and/or bulimia appeared.[32]
Precautions for use
Warnings and precautions before taking amineptine:[33]
- Breast feeding
- Children less than 15 year of age
- General anaesthesia: Discontinue the drug 24 to 48 hours before anaesthesia.[citation needed]
- Official sports/Olympic Games: Prohibited substance.
- 7 March Official Journal 2000.
- Pregnancy (first trimester)[citation needed]
Contraindications
- Chorea
- Hypersensitivity: Known hypersensitivity to amineptine, in particular antecedents of hepatitis after dosage of the product.
- MAO inhibitors
See also
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ DE Patent 2011806 - NEW TRICYCLIC DERIVATIVES AND PROCESS FOR THEIR MANUFACTURE
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.[page needed]
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 16.0 16.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 17.0 17.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Concours Med 1982; 104:5733-5734[verification needed]
- ↑ 22.0 22.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ centres-pharmacovigilance.net
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Amineptine Medication - Uses, Side Effects and Precautions of Amineptine. Health-care-information.org. Retrieved on September 28, 2013
- Wikipedia articles needing page number citations from September 2010
- Wikipedia articles needing factual verification from December 2009
- Use dmy dates from June 2013
- Chemical articles having calculated molecular weight overwritten
- Infobox drug articles without a structure image
- Articles with unsourced statements from September 2013
- Tricyclic antidepressants
- Withdrawn drugs
- Dibenzocycloheptenes
- Laboratoires Servier
- Norepinephrine-dopamine reuptake inhibitors
- Amines
- Carboxylic acids