Ibotenic acid
 |
|
 |
|
| Names | |
|---|---|
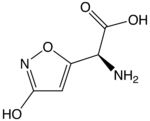
| IUPAC name
(S)-2-amino-2-(3-hydroxyisoxazol-5-yl) acetic acid
|
|
| Other names
Ibotenic acid
|
|
| Identifiers | |
| 2552-55-8 |
|
| ChEMBL | ChEMBL284895 ChEMBL30285 |
| ChemSpider | 1196 |
| 1371 | |
| Jmol 3D model | Interactive image |
| PubChem | 1233 |
|
|
|
|
| Properties | |
| C5H6N2O4 | |
| Molar mass | 158.11 g/mol |
| Melting point | 151-152° (anhydrous); 144-146° (monohydrate) |
| Solubility in Methanol | Soluble |
| Vapor pressure | {{{value}}} |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Ibotenic acid or (S)-2-amino-2-(3-hydroxyisoxazol-5-yl) acetic acid, also referred to as ibotenate, is a chemical compound and psychoactive drug which occurs naturally in Amanita muscaria and related species of mushrooms typically found in the temperate and boreal regions of the northern hemisphere. It is a conformationally-restricted analogue of the neurotransmitter glutamate, and due to its structural similarity to this neurotransmitter, acts as a non-selective glutamate receptor agonist.[1] Because of this, ibotenic acid is a powerful neurotoxin, and is employed as a "brain-lesioning agent" in scientific research.[2][3]
Contents
Pharmacology

Ibotenic acid acts as a potent agonist of the NMDA and group I (mGluR1 and mGluR5) and II (mGluR2 and mGluR3) metabotropic glutamate receptors.[1][4] It is inactive at group III mGluRs.[5] Ibotenic acid also acts as a weak agonist of the AMPA and kainate receptors.[1][4] In addition, due to in vivo decarboxylation into muscimol, it acts indirectly as a potent GABAA and GABAA-ρ receptor agonist.[4] Unlike muscimol, the principal psychoactive constituent of Amanita muscaria that is understood to cause sedation and dissociation, ibotenic acid's psychoactive effects are not known independent of it serving as a prodrug to muscimol.[6][7]
Biological properties
Mechanism of action
Ibotenic acid is an agonist of glutamate, specifically at both the N-methyl-D-aspartate, or NMDA and trans-ACPD receptor sites in a multiple of systems in the central nervous system. Ibotenic neurotoxicity can be enhanced by glycine and blocked by MK-801. MK-801 acts as a competitive antagonist against the Ibotenate.[8]
Ibotenic acid toxicity comes from activation of the NMDA receptors. NMDA receptors are related to synaptic plasticity and work with metabotropic glutamate receptors to establish long term potentiation or LTP. The process of long term potentiation is believed to be related to the acquisition of information. The NMDA receptor functions properly by allowing Ca2+ ions to pass through after activation at the receptor site.
The binding of ibotenic acid allows excess Ca2+ into the system which results in neuronal cell death or apoptosis. Ca2+ also activates CaM-KII or Ca2+/Calmodulin Kinase which phosphorylates multiple enzymes. The activated enzymes then begin producing reactive oxygen species which damages surrounding tissue. The excess Ca2+ results in the enhancement of the mitochondrial electron transport system which will further increase the number of reactive oxygen species.[9]
Biological effects
Ibotenic acid typically affects NMDA and trans-ACPD receptors in the central nervous system.[8] Due to their targeting of these systems the symptoms associated with Ibotenic acid poisoning are often related to perception and control.
Symptoms associated with the compound are usually onset within 30–60 minutes and include a range of nervous system effects. The most common symptoms include, nausea, vomiting, and drowsiness. However, after the first hour symptoms begin to include confusion, euphoria, visual and auditory distortions, sensations of floating, and retrograde amnesia.
Symptoms are slightly different for children, typically beginning after 30–180 minutes. Dominant symptoms in children include ataxia, obtundation, and lethargy. Seizures are occasionally reported, however, more commonly with children.[10]
Treatment

Treatment of ibotenic acid poisoning is limited and varies as the toxic dose of the compound varies from person to person. Patients admitted to the hospital for ibotenic acid poisoning are typically given charcoal in order to stop the absorption of the compound and prevent further intoxication. Upon receiving charcoal treatment the patient’s vital signs are monitored and toxicity usually lasts between 6 and 8 hours; however, some symptoms may take up to a few days to subside. Occasionally, to reduce the discomfort associated with symptoms, benzodiazepines will be administered to control panic attacks and hallucinations.
Special attention should be given to monitoring breathing, airway control, and circulation. If treatment occurs within the first hour of ingestion gastric lavage may be effective. If vomiting becomes too intense intravenous fluids are administered, psychiatric care may also be provided.
In rare cases anticholinergic drugs, such as atropine, may be needed.[11]
Use in research
Ibotenic acid used for the lesioning of rat's brains is kept frozen in a phosphate-buffered saline solution at a pH of 7.4 and can be kept for up to a year with no loss in toxicity. Injection of .05-.1 microliters of Ibotenic acid into the hippocampus at a rate of .1 microliter/min resulted in semi-selective lesioning. Hippocampal lesioning led to a considerable loss of cells in pyramidal cells (CA1-CA3) as well as granule cells in the dentate gyrus. Ibotenic acid lesioning also causes some damage to axons along the perforant pathway.
Typically when lesioning is done with other chemicals the subject can not relearn a task. However, due to Ibotenic acid's reactivity with glutamate receptor's such as NDMA, Ibotenic acid lesioning does allow the subject to relearn tasks. Due to this, Ibotenic acid lesioning is preferred in studies where re-learning a task after lesioning is essential. When compared to other lesioning agents, Ibotenic acid is one of the most site-specific, however, on going research is searching for less-damaging alternatives.[12]
See also
References
- ↑ 1.0 1.1 1.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 4.0 4.1 4.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Chilton 1975; Theobald et al. 1968
- ↑ Chilton 1975; Ott 1976a
- ↑ 8.0 8.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
Lua error in package.lua at line 80: module 'strict' not found.
- Articles without KEGG source
- Articles without UNII source
- Pages using collapsible list with both background and text-align in titlestyle
- Chemical articles using a fixed chemical formula
- Amino acids
- AMPA receptor agonists
- GABAA receptor agonists
- GABAA-rho receptor agonists
- Kainate receptor agonists
- MGlu1 receptor agonists
- MGlu2 receptor agonists
- MGlu3 receptor agonists
- MGlu5 receptor agonists
- NMDA receptor agonists
- Isoxazoles
- Mycotoxins found in Basidiomycota
- Neurotoxins
- Prodrugs
- Psychedelic drugs


