Glaucine
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
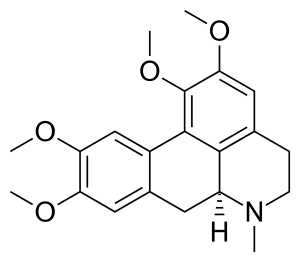
(S)-5,6,6a,7-tetrahydro-1,2,9,10-tetramethoxy-6-methyl-4H-dibenzo[de,g]quinoline
|
|
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Identifiers | |
| CAS Number | 475-81-0 Template:CAS |
| ATC code | none |
| PubChem | CID: 16754 |
| ChemSpider | 15883 |
| UNII | NU19306XA7 |
| KEGG | D08014 |
| ChEMBL | CHEMBL228082 |
| NIAID ChemDB | 011385 |
| Chemical data | |
| Formula | C21H25NO4 |
| Molecular mass | 355.428 g/mol |
|
|
|
|
| |
|
Glaucine is an alkaloid found in several different plant species in the Papaveraceae family such as Glaucium flavum,[1] Glaucium oxylobum and Corydalis yanhusuo,[2][3] and in other plants like Croton lechleri in the family Euphorbiaceae.[4]
It has bronchodilator and antiinflammatory effects, acting as a PDE4 inhibitor and calcium channel blocker,[5] and is used medically as an antitussive in some countries.[6] Glaucine may produce side effects such as sedation, fatigue, and a hallucinogenic effect characterised by colourful visual images,[7][8] and has been detected as a novel psychoactive drug.[9]
Contents
Mechanism of action
Glaucine binds to the benzothiazepine site on L-type Ca2+-channels, thereby blocking calcium ion channels in smooth muscle like the human bronchus. Glaucine has no effect on intracellular calcium stores, but rather, does not allow the entry of Ca2+ after intracellular stores have been depleted.[5] Ca2+ influx is a vital component in the process of muscular contraction, and the blocking of this influx therefore reduces the ability of the muscle to contract.[10] In this way, glaucine can prevent smooth muscle from contracting, allowing it to relax.
Glaucine has also been demonstrated to be a dopamine receptor antagonist, favoring D1 and D1-like receptors.[11][9] It is also a non-competitive selective inhibitor of PDE4 in human bronchial tissue and granulocytes. PDE4 is an isoenzyme that hydrolyzes cyclic AMP to regulate human bronchial tone (along with PDE3). Yet as a PDE4 inhibitor, glaucine possesses very low potency.[5]
Clinical use
It is currently used as an antitussive agent in Iceland, as well as Romania, Bulgaria, Russia and other eastern European countries.[5][9] Bulgarian pharmaceutical company Sopharma sells glaucine in tablet form, where a single dose contains 40 mg and the half-life is indicated to be 6–8 hours. When ingested orally has been shown to increase airway conductance in humans, and has been investigated as a treatment for asthma.[5]
Animal studies demonstrate the ability of glaucine to decrease heart rate and lower blood pressure,[12] presumably by the same mechanism of Ca2+-channel antagonism that it uses to relax bronchial muscle. Studies of the effect of several alkaloids in mice, including glaucine, demonstrate anticonvulsant and antinociceptive properties.[13] In other words; animal studies indicate that glaucine can also act as a pain reliever to a certain extent, although its capacities in this respect appear limited when compared to other analgesics.
Symptoms and recreational use
Reports of recreational use of glaucine have recently been published, and effects include dissociative-type symptoms; feeling detached and ‘in another world’, as well as nausea, vomiting and dilated pupils. These reports mirror those about the effects of clinical use, which state dissociative-type symptoms as well as lethargy, fatigue, hallucinations.[9][8] Investigation of side effects in a clinical setting also reports that the hallucinatory effects manifest as bright and colorful visualizations. They also report that patients perceive their environments clearly yet feel detached from it; “the patient sees and understands everything and is oriented well enough, but cannot take a clear and adequate action”.[8]
One particular report of recreational use gone awry described the form of distribution as tablets being marketed as a 1-benzylpiperazine (BZP)-free “herbal high” which the patient referred to as “head candy”.[9]
See also
- Apomorphine
- Bulbocapnine
- Nantenine
- Nuciferine
- Pukateine
- Stepholidine
- Tetrahydropalmatine
- Drotaverine
References
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 5.0 5.1 5.2 5.3 5.4 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 8.0 8.1 8.2 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 9.0 9.1 9.2 9.3 9.4 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Nestler E, Hyman S & Malenka R. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). China: McGraw-Hill Companies.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- Chemical articles having calculated molecular weight overwritten
- Articles with changed InChI identifier
- Infobox drug articles without a structure image
- Chemical pages without DrugBank identifier
- Drugs not assigned an ATC code
- Drugs with no legal status
- Alkaloids
- Alkaloids found in Euphorbiaceae
- Alkaloids found in Papaveraceae
- Antitussives
- PDE4 inhibitors
- Phenol ethers