Phenothiazine
 |
|
 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
10H-phenothiazine
|
|
| Other names
thiodiphenylamine, dibenzothiazine, dibenzoparathiazine, 10H-dibenzo-[b,e]-1,4-thiazine, PTZ
|
|
| Identifiers | |
| 92-84-2 |
|
| ChEBI | CHEBI:37931 |
| ChEMBL | ChEMBL828 |
| ChemSpider | 21106365 |
| Jmol 3D model | Interactive image |
| KEGG | D02601 |
| UNII | GS9EX7QNU6 |
|
|
|
|
| Properties | |
| C12H9NS | |
| Molar mass | 199.27 g/mol |
| Appearance | greenish-yellow rhombic leaflets or diamond-shaped plates |
| Melting point | 185 °C (365 °F; 458 K) |
| Boiling point | 371 °C (700 °F; 644 K) |
| 0.00051 g/L (20 °C)[1] | |
| Solubility in other solvents | benzene, ether, petroleum ether, chloroform, hot acetic acid, ethanol (slightly), mineral oil (slightly) |
| Acidity (pKa) | approx 23 in DMSO |
| Vapor pressure | {{{value}}} |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
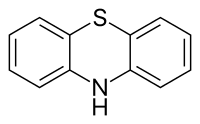
Phenothiazine is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds.
It is used in chemical manufacturing as a stabilizer or inhibitor. It was used in the mid-20th century as an insecticide and antihelminthic for livestock and humans, but was superseded by other compounds.
Derivatives of phenothiazine discovered in the 1940s revolutionized the field of psychiatry and allergy treatment. The earliest derivative, methylene blue, was one of the first antimalarial drugs, and as of 2015 derivatives are under investigation as possible anti-infective drugs. It is a prototypical pharmaceutical lead structure in modern medicinal chemistry.
Contents
Use
In the manufacture of monomers, phenothiazine is used as a chemical stabilizer or inhibitor to prolong storage and shelf life of products such as acryloyl chloride.[2][3]
Former uses
Phenothiazine was formerly used as an insecticide and as a drug to treat infections with parasitic worms (antihelminthic) in livestock and people, but its use for those purposes has been superseded by other chemicals.
Phenothiazine was introduced by DuPont as an insecticide in 1935.[4] While about 3,500,000 pounds were sold in the US in 1944,[5] However, because it was degraded by sunlight and air, it was difficult to determine how much to use in the field, and its use waned in the 1940s with the arrival of new pesticides like DDT that were more durable.[6]:161–162 As of July 2015 it is not registered for pesticide use in the US,[7] nor Europe, [3] nor Australia.[8]
It was introduced as antihelminthic in livestock in 1940 and is considered, with thiabendazole, to be the first modern antihelminthic.[9] The first instances of resistant were noted in 1961.[9] Uses for this purpose in the US are still described[10] but it has "virtually disappeared from the market."[11]:369
In the 1940s it also was introduced as antihelminthic for humans; since it was often given to children, the drug was often sold in chocolate, leading to the popular name, "worm chocolate." Phenothiazine was superseded by other drugs in the 1950s.[12]
Synthesis
The compound was originally prepared by Bernthsen in 1883 via the reaction of diphenylamine with sulfur, but more recent syntheses rely on the cyclization of 2-substituted diphenyl sulfides. Some of the pharmaceutically significant derivatives of phenothiazine are not prepared directly from phenothiazine,[13] although some of them are.[14]
Derivatives
Phenothiazine itself was a pioneering compound, but its derivatives revolutionized psychiatry and other fields of medicine. Other derivatives have been studied for possible use in advanced batteries and fuel cells.[12]
Phenothiazine-derived drugs
Methylene blue is technically a derivative of phenothiazine but was synthesized first; it was made in 1876 by Heinrich Caro at BASF and the structure was determined in 1885 by Heinrich August Bernthsen. Bernthsen synthesized phenothiazine in 1883.[12]
In the mid 1880s, Paul Ehrlich began to use methylene blue in his cell staining experiments that led to pioneering discoveries about different cell types; he won a Nobel Prize based in part of that work. He became particularly interested in its use to stain bacteria, which he pursued into other organisms like Plasmodiidae – which includes the malaria pathogen – and found that it could be stained with methylene blue. He thought methylene blue could possibly be used in the treatment of malaria, tested it clinically, and by the 1890s methylene blue was being used for that purpose.[12]
Research on derivatives lapsed until phenothiazine itself came to market as an insecticide and deworming drug. In the 1940s, chemists working with Paul Charpentier at Rhone-Poulenc Laboratories in Paris (a precursor company to Sanofi, began making derivatives. This work led to promethazine which had no activity against infective organisms, but did have good antihistamine activity, with a strong sedative effect. It went to market as a drug for allergies and for anesthesia. As of 2012 it was still on the market.[12] At the end of the 1940s the same lab produced chlorpromazine which had an even stronger sedative and soothing effect, and Jean Delay and Pierre Deniker attempted to use it on their psychiatric patients, publishing their results in the early 1950s. The strong effects they found opened the door of the modern field of psychiatry and led to a proliferation of work on phenothiazine derivatives.[12] The systematic research conducted by chemists to explore phenothiazine derivatives and their activity using was a pioneering example of medicinal chemistry; phenothiazine is often discussed as a prototypical example of a pharmaceutical lead structure.[12][15]
Today, the term "phenothiazines" describes the largest of the five main classes of antipsychotic drugs. These drugs have antipsychotic and, often, antiemetic properties, although they may also cause severe side effects such as extrapyramidal symptoms (including akathisia and tardive dyskinesia), hyperprolactinaemia, and the rare but potentially fatal neuroleptic malignant syndrome, as well as substantial weight gain.[12] Use of phenothiazines has been associated with antiphospholipid syndrome, but no causal relationship has been established.[16]
Phenothiazine antipsychotics are classified into three groups that differ with respect to the substituent on nitrogen: the aliphatic compounds (bearing acyclic groups), the "piperidines" (bearing piperidine-derived groups), and the piperazine (bearing piperazine-derived substituents).[15]
| Group | Autonomic | Example | Sedative | Extrapyramidal side effects |
| Aliphatic compounds | ||||
| moderate | Chlorpromazine (marketed as Thorazine, Aminazine, Chlor-PZ, Klorazine, Promachlor, Promapar, Sonazine, Chlorprom, Chlor-Promanyl, Largactil) | strong | moderate | |
| Promazine (trade name Sparine, Propazine) | moderate | moderate | ||
| Triflupromazine (trade names Clinazine, Novaflurazine, Pentazine, Terfluzine, Triflurin, Vesprin) | strong | moderate/strong | ||
| Levomepromazine in Germany, Russia and methotrimeprazine in America (trade names Nozinan, Levoprome, Tisercin) | extremely strong | low | ||
| Piperidines | strong | Mesoridazine (trade name Serentil) | strong | weak |
| Thioridazine (trade names Mellaril, Novoridazine, Thioril, Sonapax) | strong | weak | ||
| Piperazines | weak | Fluphenazine (trade names Prolixin, Permitil, Modecate, Moditen) | weak/moderate | strong |
| Perphenazine (sold as Trilafon, Etrafon, Triavil, Phenazine, Etaperazin) | weak/moderate | strong | ||
| Prochlorperazine (trade names Compazine, Stemetil) | ||||
| Trifluoperazine (trade name Stelazine, Triphtazine) | moderate | strong |
Nondrug applications
The synthetic dye methylene blue, containing the structure, was described in 1876. Many water-soluble phenothiazine derivatives, such as methylene blue, methylene green, thionine, and others, can be electropolymerized into conductive polymers used as electrocatalysts for NADH oxidation in enzymatic biosensors and biofuel cells.[17][18][19]
References
- ↑ Sigma-Aldrich catalog
- ↑ PTZ Technical Information Bulletin Cytec PTZ Technical Information Bulletin
- ↑ 3.0 3.1 ECHA phenothiazine at the European Chemicals Authority Page accessed July 26, 2015. Note - Registered uses are only in manufacturing.
- ↑ History of Insecticides and Control Equipment Clemson University Pesticide Information Program.
- ↑ Robert Lee Metcalf. The Mode of Action of Organic Insecticides, Issues 1-5. National Academies, 1948, page 44
- ↑ G. Matolcsy, M. Nádasy, V. Andriska. Studies in Environmental Science: Pesticide Chemistry. Elsevier, 1989 ISBN 9780080874913
- ↑ Phenothiazine in the PAN Pesticides Database Page accessed July 26, 2015
- ↑ Australian Pesticides and Veterinary Medicine Authority Phenothiazine Chemical Review Page accessed July 26, 2015
- ↑ 9.0 9.1 Nielsen MK, et al. Anthelmintic resistance in equine parasites--current evidence and knowledge gaps. Vet Parasitol. 2014 Jul 30;204(1-2):55-63. Review. PMID 24433852
- ↑ The Texas A&M University System; Texas AgriLife Extension Service Integrated pest management of �flies in Texas dairies
- ↑ Heinz Mehlhorn, Philip M. Armstrong. Encyclopedic Reference of Parasitology: Diseases, Treatment, Therapy, Volume 2. Springer Science & Business Media, 2001 ISBN 9783540668299
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 Ohlow MJ, Moosmann B. Phenothiazine: the seven lives of pharmacology's first lead structure. Drug Discov Today. 2011 Feb;16(3-4):119-31. PMID 21237283
- ↑ Gérard Taurand, "Phenothiazine and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a19_387
- ↑ T. Kahl, K.-W. Schröder, F. R. Lawrence, W. J. Marshall, Hartmut Höke, Rudolf Jäckh, "Aniline" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH: Weinheim.
- ↑ 15.0 15.1 Jaszczyszyn A, et al. Chemical structure of phenothiazines and their biological activity. Pharmacol Rep. 2012;64(1):16-23. Review. PMID 22580516
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
Further reading
- MSDS
- Hendricks, Christensen, J.B., and Kristiansen, Jette E. Sonderborg, Denmark. "Antibakterielle Eigenschaften der Phenothiazine: Eine Behandlungsoption für die Zukunft?" Chemotherapie Journal. 13.5. (2004): 203–205. Wissenschaftliche Verlagsgesesellschaft mbH. 21 August 2005. (PDF).
- PubChem Substance Summary: Phenothiazine National Center for Biotechnology Information.
- CDC - NIOSH Pocket Guide to Chemical Hazards