Flupirtine
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
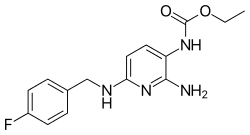
ethyl {2-amino-6-[(4-fluorobenzyl)amino]pyridin-3-yl}carbamate
|
|
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Pharmacokinetic data | |
| Bioavailability | 90% (oral), 70% (rectal)[1] |
| Metabolism | Hepatic to 2-amino-3-acetylamino-6-(para-fluorobenzylamino) pyridine (which has 20-30% the analgesic potential of its parent compound), para-fluorohippuric acid[2] and a mercapturic acid metabolite, presumably formed from a glutathione adduct[3] |
| Biological half-life | 6.5 hrs (average), 11.2-16.8 hrs (average 14 hrs) (elderly), 8.7-10.9 hrs (average 9.8 hrs) (in those with moderate-level renal impairment)[1] |
| Excretion | 72% of flupirtine and its metabolites appear in urine and 18% appear in feces[4] |
| Identifiers | |
| CAS Number | 56995-20-1 |
| ATC code | N02BG07 (WHO) |
| PubChem | CID: 53276 |
| IUPHAR/BPS | 2598 |
| ChemSpider | 48119 |
| UNII | MOH3ET196H |
| KEGG | D07978 |
| ChEMBL | CHEMBL255044 |
| Chemical data | |
| Formula | C15H17FN4O2 |
| Molecular mass | 304.32 g/mol |
|
|
|
|
| |
|
Flupirtine is an aminopyridine that functions as a centrally acting non-opioid analgesic. It first became available in Europe in 1984, and is sold mainly under the names Katadolon, Trancolong, Awegal, Efiret, Trancopal Dolo, and Metanor.[5] Like nefopam, it is unique among analgesics in that it is a non-opioid, non-NSAID, non-steroidal centrally acting analgesic. In 2010 the chemically related drug (the difference being that the pyridine group in flupirtine is replaced with a phenyl group) retigabine (INN; ezogabine [USAN]) was approved by the FDA as an anticonvulsant for the treatment of refractory partial-onset seizures in treatment-experienced patients.[6] Retigabine also works by opening the neuronal KCNQ/Kv7 potassium channel, just like flupirtine.
History
Flupirtine was originally developed by Asta Medica, with the synthesis of the compound and the development of the drug described in patents from the 1970s to the 2000s.[7][8][9][10][11][12]
It was approved for the treatment of pain in 1984 in Europe. However, it has never been introduced to the United States market for any indication. In 2008, Adeona Pharmaceuticals, Inc. (now called Synthetic Biologics, Inc.) obtained an option to license issued and patent pending applications relating to flupirtine’s use in the treatment of ophthalmic indications, particularly retinitis pigmentosa.[13]
Mechanism of Action
Flupirtine is a selective neuronal potassium channel opener that also has NMDA receptor antagonist and GABAA receptor modulatory properties.[14]
Uses
Flupirtine is used as an analgesic for acute and chronic pain, in moderate-to-severe cases.[15] Its muscle relaxant properties make it popular for back pain and other orthopedic uses, but it is also used for migraines, in oncology, postoperative care, and gynecology.
Flupirtine has been noted for its neuroprotective properties, and it is being investigated for possible use in Creutzfeldt–Jakob disease, Alzheimer's disease, and multiple sclerosis.[16][17] It has also been proposed as a possible treatment for Batten disease.[18]
Flupirtine underwent a clinical trial as a treatment for multiple sclerosis[19] and fibromyalgia.[20] Flupirtine showed promise for fibromyalgia due to its different action than the three approved by U.S. FDA drugs: Lyrica (pregabalin), Savella (milnacipran), and Cymbalta (duloxetine).[21] Additionally, there are case reports regarding flupirtine as a treatment for fibromyalgia.[22] Adeona Pharmaceuticals (now called Synthetic Biologics) sub-licensed its patents for using flupirtine for fibromyalgia to Meda AB in May 2010.[21]
Side Effects
The most serious side effect is frequent hepatotoxicity which prompted regulatory agencies to issue several warnings and restrictions.[23][24]
Flupirtine is devoid of negative psychological or motor function effects, or effects on reproductive function.[25][26]
Abuse and Dependence
Although some studies have reported flupirtine has no addictive properties,[27][28] there was suggestion that it may possess some abuse potential and liability.[29] There were at least two registered cases of flupirtine abuse.[30] Drug tolerance does not develop in most cases; however, tolerance may develop in single cases.[30]
References
- ↑ 1.0 1.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Flupirtine Drugs.com. Accessed 20 September 2011.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ http://www.freepatentsonline.com/5721258.html Primary and secondary neuroprotective effect of flupirtine in neurodegenerative diseases The synthesis of flupirtine and its pharmaceutically acceptable salts is described in EP 160 865 and 199 951. EP0199951 December, 1986 Process for the preparation of 2-amino-3-nitro-6-(4-fluorobenzylamino) pyridine and of 2-amino-3-carbethoxyamino-6-(4-fluorobenzylamino) pyridine.
- ↑ http://patent.ipexl.com/EP/EP0199951.html#reference Process for the preparation of 2-amino-3-nitro-6-(4-fluorobenzylamino) pyridine and of EPO Patent EP0199951 1986 German.
- ↑ http://www.patentfish.com/2-amino-3-carbethoxyamino-6-4-fluoro-benzylamino Process for the preparation of 2-amino-3-nitro-6-(4-fluorobenzylamino) pyridine and of 2-amino-3-carbethoxyamino-6-(4-fluorobenzylamino) pyridine EP 0199951 B1 1986. English.
- ↑ http://patent.ipexl.com/US/4481205.html 2-Amino-3-carbethoxyamino-6-(p-fluoro-benzylamino)-pyridine-maleate United States Patent 4481205. 1981
- ↑ http://www.freepatentsonline.com/3998834.html Novel N-(4-piperidinyl)-N-phenylamides and -carbamates having very potent analgesic activity, methods of preparing same and useful intermediates therefor. Patent 3998834. 1976.
- ↑ http://www.faqs.org/patents/app/20090306150 CARBOXYLIC ACID SALTS OF 2-AMINO-3-CARBETHOXYAMINO-6-(4-FLUORO-BENZYLAMINO)-PYRIDINE patent 20090306150. 2009. Flupirtine is commonly used in the form of pharmaceutically acceptable acid addition salts. Commercially, flupirtine is available as its maleate addition salt under the trademark Katadolon®. There are two known polymorphs of flupirtine maleate, designated in the art as flupirtine maleate A and B. European patentEP 0 977 736 discloses pure flupirtine maleate crystalline form A and a process for its preparation. Flupirtine and mixtures of flupirtine maleate polymorphs A and B can be synthesised according to EP 0 199 951.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Flupirtine as Oral Treatment in Multiple Sclerosis (FLORIMS) Clinical Trials.gov Accessed 20 September 2011.
- ↑ Pipex Pharmaceuticals (PPXP)' Oral Flupirtine Receives IND With FDA for Phase II Clinical Trial for Fibromyalgia 4/21/2008
- ↑ 21.0 21.1 Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Stoll AL, Belmont MA. (2000) "Fibromyalgia Symptoms Relieved by Flupirtine: An Open-Label Case Series" Psychosomatics 41:371-372. Accessed 20 September 2011.
- ↑ EMA information about flupirtine
- ↑ article in Deutsches Ärzteblatt
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ Lua error in package.lua at line 80: module 'strict' not found.
- ↑ 30.0 30.1 Lua error in package.lua at line 80: module 'strict' not found.
- Chemical articles having calculated molecular weight overwritten
- Articles with changed InChI identifier
- Infobox drug articles without a structure image
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Pyridines
- Carbamates
- Potassium channel openers
- Organofluorides
- GABAA receptor positive allosteric modulators
- NMDA receptor antagonists